Table 1. Glucose Building Blocks and Solution Phase Glycosylationsa.
| entry | building block | acceptor | ratio (α/β) | yield (α + β) | entry | building block | acceptor | ratio (α/β) | yield (α + β) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | i-PrOH | 1:2.9 | 93% | 15 | 12 | 33 | 1.5:1 | 83% |
| 2 | 13 | i-PrOH | 2.3:1 | 95% | 16 | 13 | 33 | 3.8:1 | 75% |
| 3 | 17 | i-PrOH | 2.5:1 | 92% | 17 | 17 | 33 | 5.5:1 | 69% |
| 4 | 20 | i-PrOH | 1.7:1 | 93% | 18 | 20 | 33 | 6.8:1 | 72% |
| 5 | 22 | i-PrOH | 2.2:1 | 90% | 19 | 22 | 33 | 5.6:1 | 68% |
| 6 | 23 | i-PrOH | 3.5:1 | 89% | 20 | 23 | 33 | 6.2:1 | 67% |
| 7 | 25 | i-PrOH | 1.9:1 | 90% | 21 | 25 | 33 | 7.5:1 | 44% |
| 8 | 26 | i-PrOH | 4.5:1 | 84% | 22 | 26 | 33 | 9.8:1 | 78% |
| 9 | 27 | i-PrOH | 6.4:1 | 88% | 23 | 27 | 33 | >10:1 | 62% |
| 10 | 28 | i-PrOH | 4.8:1 | 87% | 24 | 28 | 33 | >10:1 | 83% |
| 11 | 29 | i-PrOH | 7.1:1 | 74% | 25 | 29 | 33 | >10:1 | 64% |
| 12 | 30 | i-PrOH | 2.4:1 | 76% | 26 | 30 | 33 | >10:1 | 80% |
| 13 | 31 | i-PrOH | 1:1.1 | 96% | 27 | 31 | 33 | 3.9:1 | 75% |
| 14 | 32 | i-PrOH | 1:2.6 | 94% | 28 | 32 | 33 | 2.7:1 | 81% |
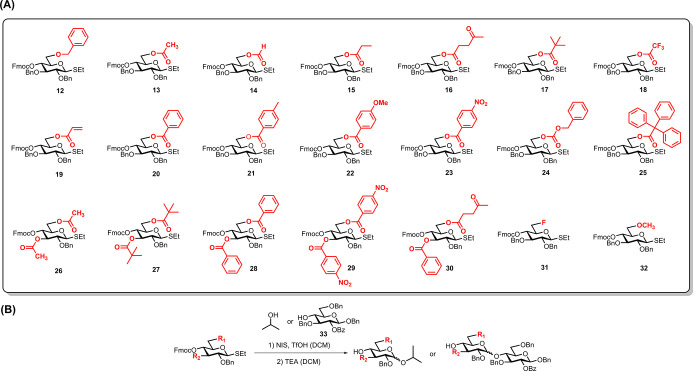
(A) The glucose thioglycoside building blocks were prepared from a common precursor as described in Figure S1. (B) Glycosylations using isopropanol and monosaccharide as nucleophiles. The ratio of α/β anomers were quantified by NMR, yield represented the isolated yield of α- and β-anomers (complete results in Table S1).