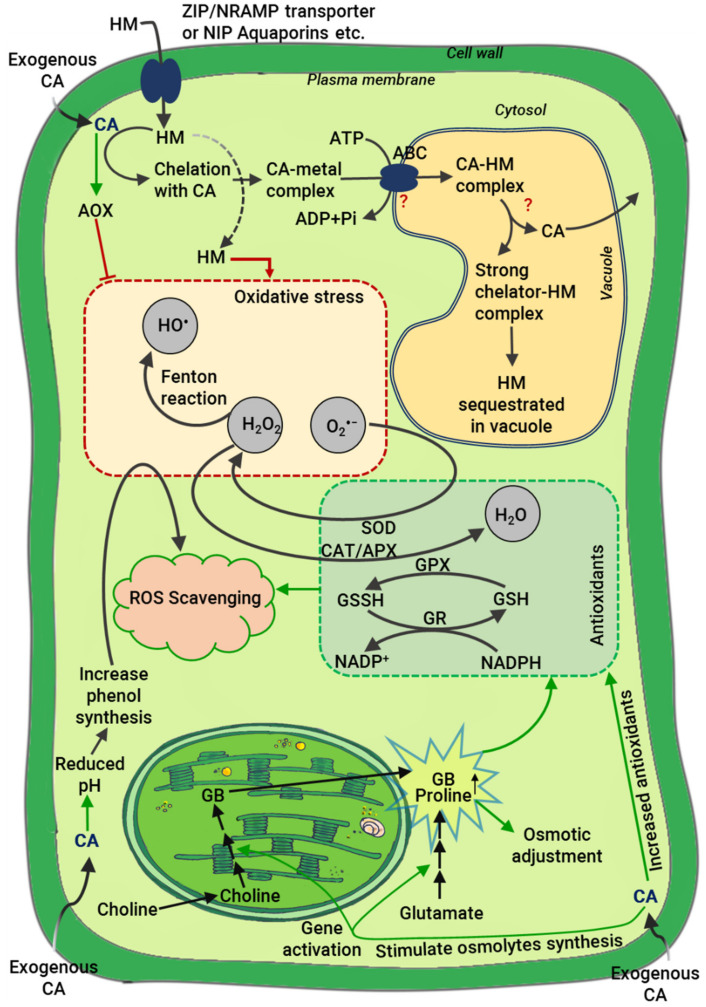
Figure 3.
Overview of cellular mechanisms for HM detoxification and stress tolerance involving citric acid (CA). HMs enter cytosol after uptake through anion channels or metal transporters, for example, ZIP (zinc/iron -regulated transporter) family members or NRAMPs (macrophage proteins associated with natural resistance) family members or NIP aquaporin (nodulin-26-like intrinsic proteins of the aquaporin family) etc. Cellular CA functions as high-affinity ligand, chelating HMs in the cytosol and then binding together to form a stable chelation complex via the cytosol ligand exchange reaction. The chelation complex is then transported into the vacuole via vacuolar transporters like ABC (ATP-binding cassette) tonoplast transporter achieving HM sequestration. CA further aids vacuolar compartmentalization or remobilization of HMs by buffering the concentrations of cytosolic HMs, but the precise mechanism remains unclear. HMs induce oxidative stress in cells, leading to the formation of ROS. Exogenous CA enhances antioxidant systems (e.g., glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), etc.) to fine-tune ROS levels and maintain normal cellular activities. High cellular CA also activates alternative oxidase (AOX) and that detoxifies ROS. Exogenous CA also induces osmolyte synthesis (e.g., proline, glycine betaine (GB), etc.) which regulates the osmotic balance and promotes ROS scavenging enzyme gene expression. Finally, CA decreases the pH of the cell and increases the synthesis of total polyphenol compounds (TPC) which directly scavenge ROS.