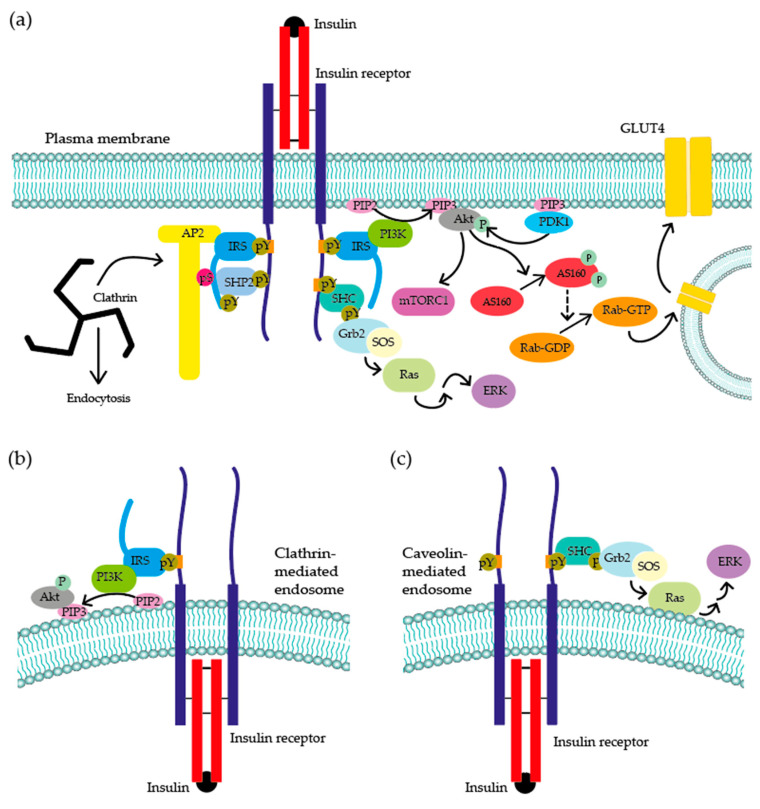
Figure 1.
Signalling cascade initiated by insulin binding to the insulin receptor (INSR). (a) After insulin binding, receptors aggregate, and autophosphorylation occurs on multiple tyrosine residues, attracting the adaptor protein SHC, which becomes phosphorylated, leading to the activation of the Ras/RAF/MEK/ERK pathway. INSR phosphorylation at Y960 recruits insulin receptor substrate 1 (IRS1), phosphorylating it on Y612, Y632 and Y662. Y612 and Y632 phosphorylation are responsible for the recruitment and activation of PI3K, which leads to Akt activation and, ultimately, to GLUT4 translocation to the plasma membrane in those cells where it is expressed. IRS-1 tyrosine phosphorylation also recruits activated ERK, which causes IRS1 serine phosphorylation, attracting the phosphatase SHP2 and resulting in IRS-1 tyrosine dephosphorylation. This promotes IRS1 interaction with AP2, triggering the internalisation of INSR [9,11]. Once internalised, receptor-mediated signalling is still maintained. Indeed, in the case of clathrin-mediated endocytosis (b), up to 50% of insulin-stimulated PI3K activity is generated from internalised receptors [6]. However, (c) in cells where INSR is internalised through caveolin-1 (e.g., pancreatic β-cells), ERK activation is enhanced [7]. This indicates that, depending on the type of endocytosis and endosomes localisation, there could be separate insulin-induced activation hubs.