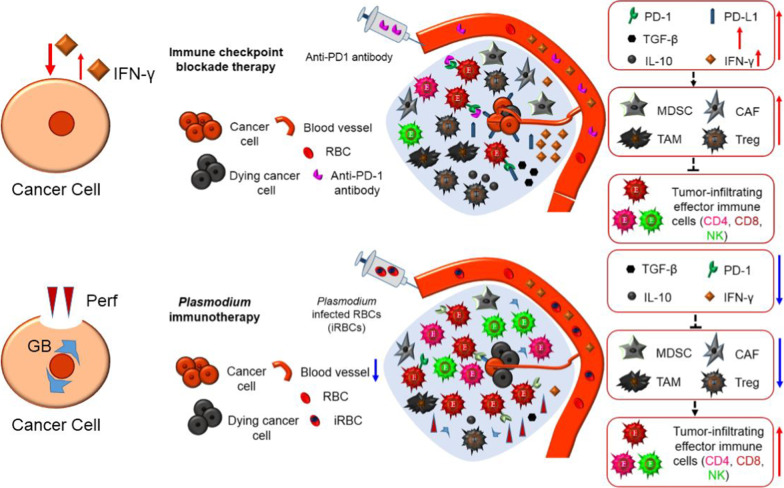
Fig. 2.
Mechanistic comparison of Plasmodium immunotherapy (down panel) with checkpoint blockade therapy (up panel). In some cancer patients, checkpoint blockade therapy are effective at the beginning, but are ineffective thereafter, this situation is called acquired drug resistance that is induced by IFN-γ secreted from reactivated CD8+ T cells. IFN-γ is a double-edged sword, it can kill cancer cells, but those that are not killed would express more PD-L1 due to the stimulation of IFN-γ, and the reactivated CD8+ T cells also express more PD-1 due to the irritation of this cytokine. Furthermore, IFN-γ is required for the development of immune suppressor cells, and for their immunosuppressive activities. Therefore, IFN-γ secreted by CD8+ T cells would increase the number of MDSCs, Tregs, TAMs and CAFs within tumor. With the recruitment of immune suppressor cells, their immunosuppressive molecules such as IL-10 and TGF-β would also increase, which in turn induce more immune suppressor cells within tumor, finally forming a vicious circle that cause the acquired drug resistance. Plasmodium immunotherapy activates the whole immune system from the beginning of innate immunity, counteract tumor immunosuppressive microenvironment, reduces the number of MDSCs, Tregs and other immunosuppressive cells, reduces the levels of their effector molecules such as TGF-β and IL-10, promotes the infiltration of immune cells into tumor, thus turn cold tumor into hot tumor, and inhibits tumor angiogenesis. Plasmodium immunotherapy stimulates CD8+ T cells to secrete perforin and granzyme B, but not secretes IFN-γ within tumor, thereby not inducing a vicious circle