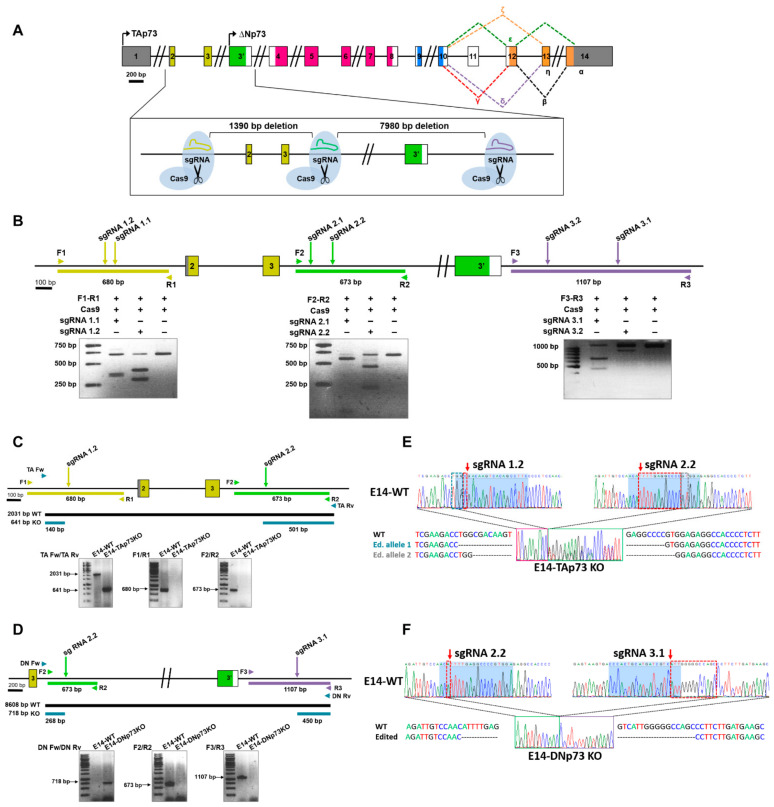
Figure 1.
Generation of TA- and DN-p73-specific knockout mESC by CRISPR/Cas9 gene editing. (A) Diagram of the followed strategy. (B) In vitro assessment of the CRISPR/Cas9-mediated cleavage. Target regions were amplified by PCR from E14TG2α genomic DNA and incubated with the Cas9 protein and the corresponding sgRNA. Cleavage locations are indicated by arrows. (C,D). The scheme indicates the expected PCR fragments (Primers Fw/Rv) to confirm correct gene editing of the E14-TA- (C) and E14-DN- (D) p73KO clones. Amplification across the boundaries of the sgRNA binding regions (Primers F1/R1, F2/R2 and F3/R3) showed the expected amplicon size for E14-WT genomic DNA, while no products were detected for the edited clones. (E,F) Sequencing electropherograms from E14-WT cells and the selected clones (E14-TA and E14-DN-p73KO, respectively). The E14-TAp73KO clone shows a 1390 bp deletion plus small different indels between both alleles in the boundaries of the deletion (E), while the E14-DNp73KO clone has a 7890 bp deletion (including a 14 bp indel, red dotted-squares), homozygous for both alleles (F). Blue-shadowed sequence corresponds to sgRNA target sequence.