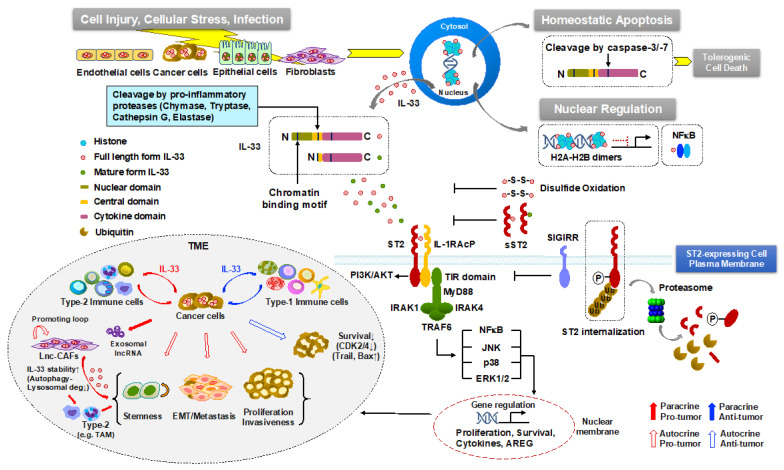
Figure 2.
The regulation of IL-33/ST2 signaling pathway. Newly synthesized full-length IL-33 remains bound to chromatin or NF-kB in the nucleus and probably acts as a suppressive nuclear factor. Upon necrosis-inducing stimuli, such as tissue damage, environmental stress, and pathogen infection, IL-33 is released to the extracellular space. The released full-length form is either retained or cleaved to produce a mature, short-form by pro-inflammatory proteases derived from mast cells and neutrophils. The full length or mature form of IL-33 binds to its receptor ST2, allowing the dimerization with the co-receptor, IL-1RAcP. This cluster recruits intracellular adaptor molecules, MyD88, IRAK1, IRAK4, and TRAF6, leading to the activation of NF-κB and MAPKs, p38, JNK, and ERK. Notably, the IL-33/ST2 axis can be negatively regulated by several distinct mechanisms. During apoptotic cell death, IL-33 is cleaved by activated apoptotic caspases. After its release, IL-33 is blocked to bind to ST2 by oxidation at cysteine residues or by binding to sST2 that functions as a decoy receptor, therefore, abrogating IL-33/ST2 signaling. An alternative mechanism involves sequential phosphorylation of ST2, its internalization, ubiquitination followed by proteasome-mediated degradation. Additionally, the TIR domain of SIGIRR, a negative regulator, interacts with ST2 and forms a receptor complex, inhibiting the IL-33/ST2 signaling pathway. Overall, the biological consequence of IL-33/ST2 signaling on tumorigenesis and cancer treatment is complex and dictated by the site of expression, local concentration, and distribution of different IL-33 isoforms and their receptors (ST2L and sST) together with the main types of responsive cells in both autocrine and paracrine manners in the TME.