Figure 8.
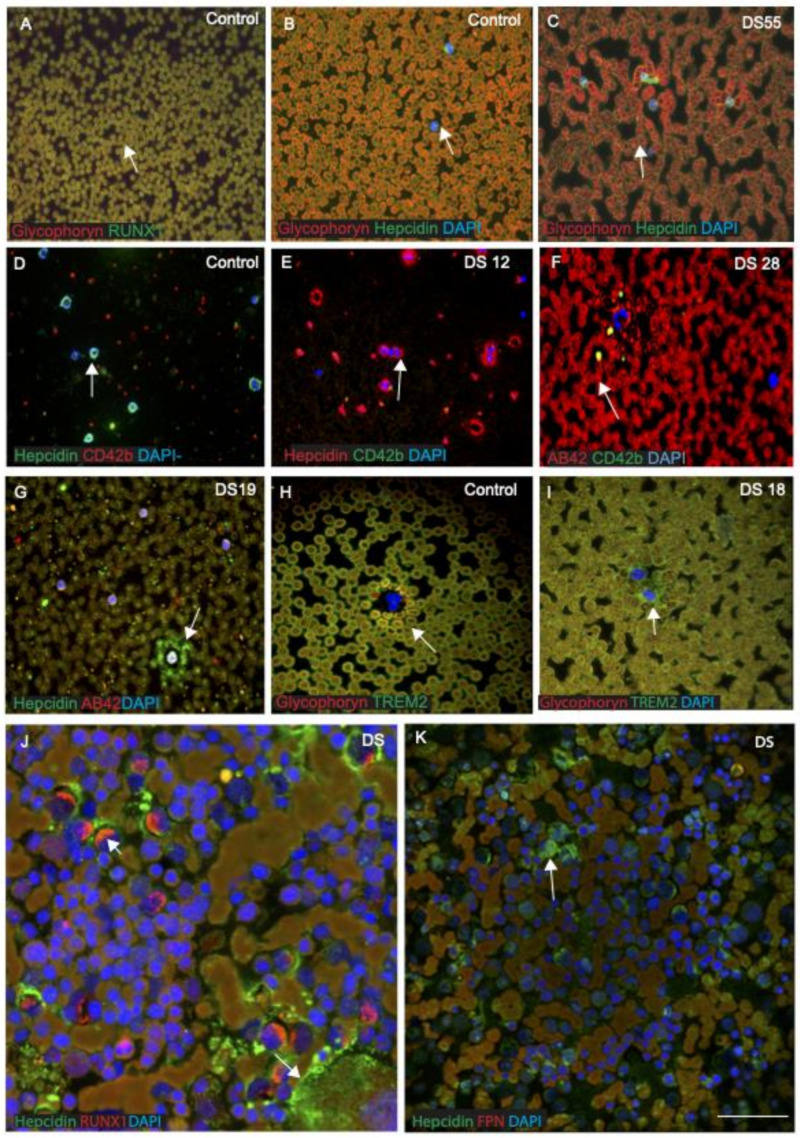
RUNX1 and hepcidin were observed in the erythro-megakaryocytes and platelets, suggesting a myeloid origin. We analysed RUNX1 and hepcidin expression in blood smears from DS subjects (n = 20) and age-matched controls (n = 20) and then imaged with confocal microscopy. DS and control blood smears were co-labelled with RUNX1 or hepcidin and glycophorin, an erythrocyte plasma membrane-marker. Both proteins (RUNX1 and glycophorin) were found to co-localise in the RBC membranes, suggesting that RUNX1 expressed on the surface of RBCs (A). Similarly, another smear from control and DS was stained with hepcidin and glycophorin. In control subjects, hepcidin was observed scattered, extra-corporally to RBCs and within the cell bodies of mononucleated cells (MNCs) (B). In young DS blood smears, a classic dysmorphology (bi-lobed neutrophils) with significantly higher hepcidin protein around the MNCs was frequently observed (C,E), whereas normal neutrophils positive for hepcidin (green) and platelet marker CD42b (red) were found in controls smears (D). The blood smear from a young DS subject (DS12) showed more hepcidin protein expression within MNC and around the neutrophils, and a limited number of CD42b-positive platelets was seen (E). One old DS subject (DS28) showed very abnormal clumped RBCs and low platelet counts (F), whereas another young DS (DS19) displayed soluble hepcidin around the MNCs, and Aβ42 was found scattered in the platelets (G). Two young DS participants’ blood sample smears (DS18, aged 32 years, and DS55, aged 39 years, with the TREM2 R47H, T mutation) showed abnormally shaped RBCs in their smears (C), with abnormal accumulation of TREM2 around the MNC (I), as reported in our previous publication [29]. A blood smear from a control participant was stained with glycophorin and TREM2, showing TREM2 being present around the RBC membrane (H). To confirm that hepcidin and RUNX1 are expressed in human bone marrow megakaryocytes, two DS bone marrow slides were stained with RUNX1, hepcidin and FPN. Both proteins were expressed in the bone marrow blast cells and co-localised where FPN expressed in RBCs (J,K). These findings indicate that RUNX1 protein is crucial for processes such as the self-renewal of haematopoietic stem cells and differentiation of the myeloid and lymphoid cell lineages, whereas hepcidin may be essential for normal development of RBCs and platelets, as well as transporting soluble proteins (Table 3). Scale bar: (A–I) = 20 μm, (J,K) = 10 μm.