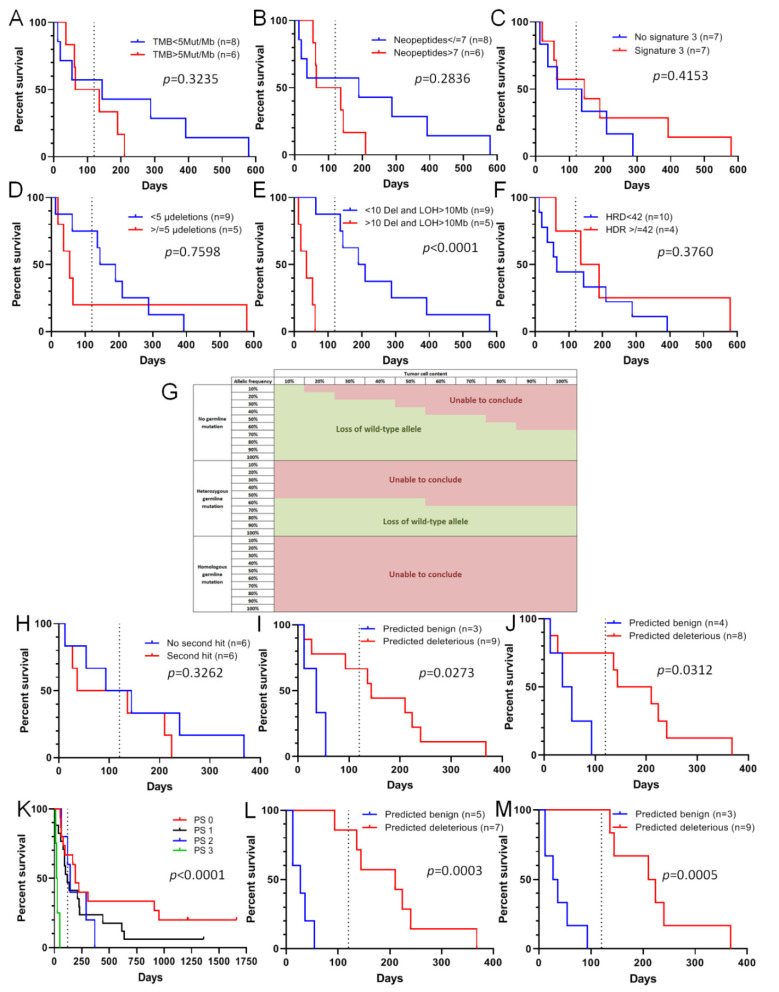
Figure 3.
Sensitivity of VUS-containing tumors to olaparib can be assessed in silico. (A–F): Progression-free survival (PFS) for patients receiving olaparib treatment according to tumor mutational burden (TMB) (A), the number of neopeptides (B), the presence of Alexandrov’s signature 3 (C), the number of microdeletions (D), homologous recombination deficiency (HRD) score (E), and the number of large deletions and loss of heterozygosity (LOH) > 10 Mb (F,G): A table enabling the determination of possible LOH depending on the tumor cell content and the allele frequency of alternative variants. Green zones correspond to putative loss of the wild-type allele. (H–M). PFS under olaparib treatment according to the presence of the second hit (H), variant pathogenicity predicted by PROVEAN (I) or Dann (J), patient Performance Status (K), variant pathogenicity predicted by combination of loss of wild-type allele, Performance status and PROVEAN (L) or Dann (M) predictions. For all panels: the dashed line corresponds to 120 days.