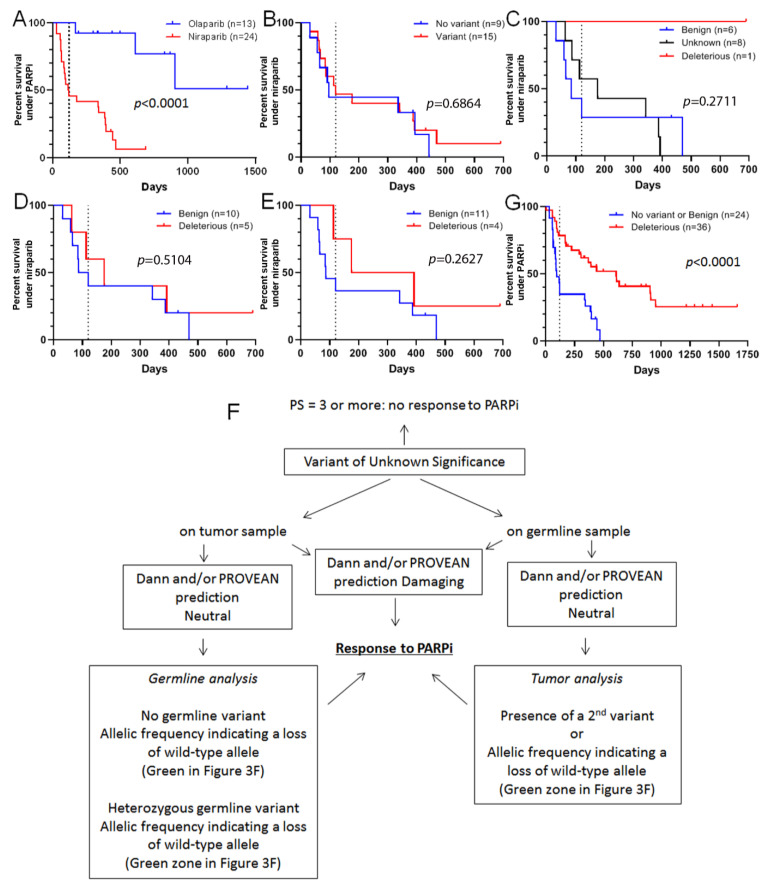
Figure 4.
Progression-free survival (PFS) of patients with ovarian cancer treated with a PARP inhibitor. (A): PFS of 37 patients prospectively treated with either olaparib (blue) or niraparib (red) in compliance with the approved indication for clinical use. (B): PFS observed for 24 patients treated with niraparib according to the absence (blue) or presence (red) of variants, whatever their pathogenicity. (C): PFS observed for patients with tumors harboring benign (blue), VUS (black) and pathogenic (red) variants in response to niraparib. (D,E): PFS under niraparib treatment according to variant pathogenicity predicted by combination of loss of wild-type allele, Performance status and Dann (D) or PROVEAN (E) predictions. (F): A proposal of a tree to guide treatment decisions in case of VUS-containing tumors. (G): PFS observed for 60 patients with ovarian cancer treated with a PARP inhibitor (olaparib or niraparib) according to the absence of a variant, or the presence of a known or predicted benign variant (blue), and the presence of a known or predicted deleterious variant (red). Only statistically significant results are indicated on the graphs. For all panels: the dashed line corresponds to 120 days.