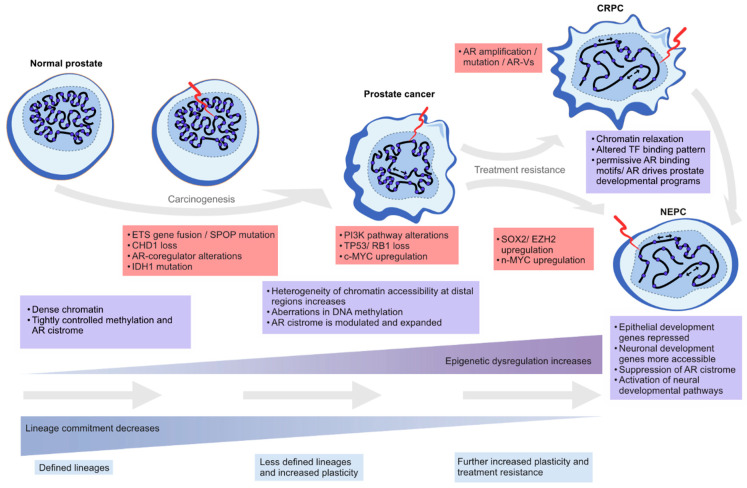
Figure 2.
Epigenetic plasticity in prostate cancer. Epigenetic dysregulation (light blue boxes) is in the forefront of lineage plasticity as well as in carcinogenesis and therapy resistance. Normal prostate epithelium is renewing at a steady state as terminally differentiated luminal cells are slowly replaced by progenitor cells. As genetic alterations accumulate due to cell division and the normal aging process, driver alterations (red boxes) such as ETS gene fusions or SPOP mutations emerge. The mutational processes lead to less ordered chromatin structure, as characterized by chromatin relaxation at distal regulatory regions, alterations in DNA methylation and histone modifications, and dysregulation of higher order chromatin structures, which alters the binding of key TFs such as AR. Some cells gain stem-like properties, leading to increased proliferation capacity and reduced apoptotic rates, which leads to tumor formation over time. The plasticity of the cellular identity is also in a key role during the emergence of treatment resistance as the cancer cells can repurpose differentiation-promoting transcription factors such as AR into regulatory regions supporting developmental gene expression (seen in castration-resistant prostate adenocarcinoma, CRPC), or transdifferentiate into non-luminal, small cell prostate carcinoma or neuroendocrine prostate cancer (NEPC).