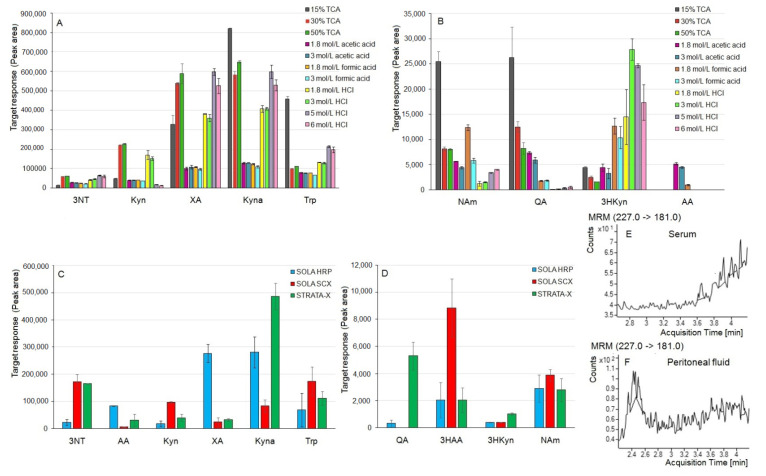
Figure 3.
Results for optimization of the serum (A,B) and peritoneal fluid preparation (C,D) for UHPLC-ESI-MS/MS analysis. (A,B) Effect of different solvents used for protein precipitation from the serum on the MS response of the target compounds. Serum (100 µL) fortified with analytes (0.5 mg/L each) was mixed with 50 µL of the precipitating solvent, which was centrifuged (14,000× g, 15 min, 4 °C), and the supernatant was transferred into a chromatographic vial and analyzed by the UHPLC-ESI-MS/MS method (injection 10 µL, 3 replicates). (C,D) Comparison of signals of analytes determined in the peritoneal fluid purified on different SPE cartridges. Applied SPE conditions: (1) STRATA-X cartridge: conditioning 1 mL MeOH, equilibration 1 mL water, loading 500 µL sample, washing 1 mL 5% (v/v) MeOH in water, elution 1 mL MeOH; (2) SOLA HRP cartridge: conditioning 500 µL MeOH, equilibration 500 µL water, loading 500 µL sample, washing 500 µL 5% (v/v) MeOH in water, elution 500 µL MeOH; (3) SOLA SCX cartridge: conditioning 500 µL MeOH, equilibration 500 µL 1% (v/v) formic acid in water, loading 500 µL sample, washing 500 µL 1% (v/v) formic acid in water followed by 500 µL 1% (v/v) formic acid in MeOH, elution 500 µL 5% (v/v) ammonium hydroxide in MeOH. The eluate was gently evaporated to dryness, redissolved in 60 µL of 5 mmol/L ammonium acetate, and analyzed by UHPLC-ESI-MS/MS (injection 5 µL, 3 replicates). Before SPE, peritoneal fluid (200 µL) spiked with analytes (12.5 mg/L 3NT, 1.25 mg/L AA, 3HAA, 3HKyn, NAm, Kyn, Trp, XA, 0.5 mg/L QA, Kyna) was mixed with 800 µL of ice-cooled 2% (v/v) acetic acid in MeOH and then centrifuged (14,000× g, 15 min, 4 °C). For data interpretation, values obtained for the control (peritoneal fluid without spike with targets) were subtracted from those for the fortified samples. The figure presents the mean values from two independent experiments. (E,F) LC–MS/MS chromatograms for MRM transition (227 > 181), selected for 3NT in the blank serum (E) and peritoneal fluid (F) from gastric cancer patients.