ABSTRACT
Understanding variation in host-associated microbial communities is important given the relevance of microbiomes to host physiology and health. Using 560 fecal samples collected from wild chimpanzees (Pan troglodytes) across their range, we assessed how geography, genetics, climate, vegetation, and diet relate to gut microbial community structure (prokaryotes, eukaryotic parasites) at multiple spatial scales. We observed a high degree of regional specificity in the microbiome composition, which was associated with host genetics, available plant foods, and potentially with cultural differences in tool use, which affect diet. Genetic differences drove community composition at large scales, while vegetation and potentially tool use drove within-region differences, likely due to their influence on diet. Unlike industrialized human populations in the United States, where regional differences in the gut microbiome are undetectable, chimpanzee gut microbiomes are far more variable across space, suggesting that technological developments have decoupled humans from their local environments, obscuring regional differences that could have been important during human evolution.
IMPORTANCE Gut microbial communities are drivers of primate physiology and health, but the factors that influence the gut microbiome in wild primate populations remain largely undetermined. We report data from a continent-wide survey of wild chimpanzee gut microbiota and highlight the effects of genetics, vegetation, and potentially even tool use at different spatial scales on the chimpanzee gut microbiome, including bacteria, archaea, and eukaryotic parasites. Microbial community dissimilarity was strongly correlated with chimpanzee population genetic dissimilarity, and vegetation composition and consumption of algae, honey, nuts, and termites were potentially associated with additional divergence in microbial communities between sampling sites. Our results suggest that host genetics, geography, and climate play a far stronger role in structuring the gut microbiome in chimpanzees than in humans.
KEYWORDS: prokaryotes, parasites, diet, tools, host genetics, climate
INTRODUCTION
Explaining differences among individuals and species in microbiome composition, including gut microbiomes, has become a key dimension of research into host-microbe interactions and the effects of changes in microbiome on host health and physiology. Such differences have the potential to influence metabolic functions, nutrition, disease resistance, and cognitive performance (1, 2). One challenging feature of this work, particularly in humans and other species in which behavior differs among populations, has been to understand the relative importance of dietary choices, environment, geography, and host genetics in structuring microbiomes. We consider these factors in light of a species-wide study of the gut microbiomes, including bacteria, archaea, and eukaryotic parasites, of wild chimpanzees (Pan troglodytes) from 29 sites in Africa ranging from 50 km to 5,130 km apart.
Perhaps the simplest explanation for variation in gut microbiome communities among individuals within a species is a model in which infants acquire microbiomes from their mothers (3, 4) and then have those microbiomes supplemented and modified via interactions with their social groups as a function of interaction frequency (5, 6) and interactions with their environment (e.g., microbial exposures in soil, food, and water) (7). This explanation predicts that microbiomes diverge among populations and lineages as a function of genetic and geographic isolation and the consequent lack of contact and opportunities for microbial transmission. Such a model reflects processes that are neutral (or indistinguishable from neutral) with regard to local adaptation. In such a model, differences in host neutral genetic divergence should be highly predictive of differences in microbiome composition. However, microbiomes might be expected to “record” differences among populations in terms of their isolation with more nuance than is seen as a function of neutral genes of hosts alone, whether due to rapid host-dependent evolutionary changes (8, 9) or changes in which different microbial taxa are extirpated from or colonize hosts (10).
Additional drivers might be expected to accelerate microbiome divergence among populations. Two related factors suggested to have strong effects on microbiomes are climate and habitat, where habitat type and specific vegetation composition are influenced by climate but also by local factors such as soil type, slope, and land use (11). It is unlikely that climate and vegetation have a direct effect on gut microbiota; few microbes in mammalian guts have life histories that allow them to survive and proliferate outside the gut, with some parasites as a notable exception. However, climate and habitat determine the foods that are available to hosts (12), the abundance and behavior of vectors for parasites (13), and the immunological condition of the hosts (14). As a result, individuals that live in different climates and habitats, with different plant species composition, are expected to have more distinct gut microbiomes, especially with respect to parasites, than would be expected based on a neutral model alone. Interestingly, humans are an exception to this case, as the important factors of diet and lifestyle can be decoupled from climate and geography, especially in industrialized societies. For example, there can be major differences in gut microbiota composition between human populations with different cultural traditions and diets, but minor differences among regions within the same cultural tradition and diet (15).
In addition to the indirect effects of climate and habitat on microbiomes via diet and host effects, there might also be effects of dietary choice on microbiomes (16). This is the case in humans, where individuals living in the same region may have different diets and, consequently, different microbiomes (1, 17, 18). A corollary of this pattern is apparent in evolutionarily distinct species that share diets and have similar gut microbiomes (19). Four chimpanzee subspecies are currently recognized (20) and inhabit a range of ecotones from dense forest to forest mosaics to savannas across equatorial Africa (Fig. 1) (21). They are considered frugivorous omnivores across their range, although forest-dwelling populations exhibit more dietary variety and frugivory than their savanna-woodland counterparts (22–24). The degree of faunivory (including insectivory) is also highly variable across the range (25, 26). However, even in similar and/or nearby environments, chimpanzees can have different diets (12, 27). This appears to be particularly common where nearby chimpanzee communities differ in their use of tools (28–33). For example, chimpanzees in Gombe National Park, Tanzania, forage on army ants (Dorylus spp.) and acrobat ants (Crematogaster spp.) using stick tools (32). In contrast, chimpanzees in Mahale Mountains National Park, less than 140 km from Gombe and with a similar habitat type in which army ants are common, do not forage on army ants but rather on carpenter ants (Camponotus spp.) (34). Similar differences are noted among chimpanzee communities in similar habitats with regard to the consumption of fruits and leaves (35), termites (31), honey (36), algae (30, 37), and some nut species that require tools to crack (29). Such behavioral differences in foraging techniques, presumed to be socially mediated and thus cultural (38), and consequent differences in diet, may in turn affect gut microbiota composition. For example, the use of tools facilitates access to dietary items that are relatively hard to digest, such as algae (those with stronger cellulose or silica cell walls), and items such as nuts that have unique protein and fat profiles relative to a predominantly fruit- and leaf-based diet.
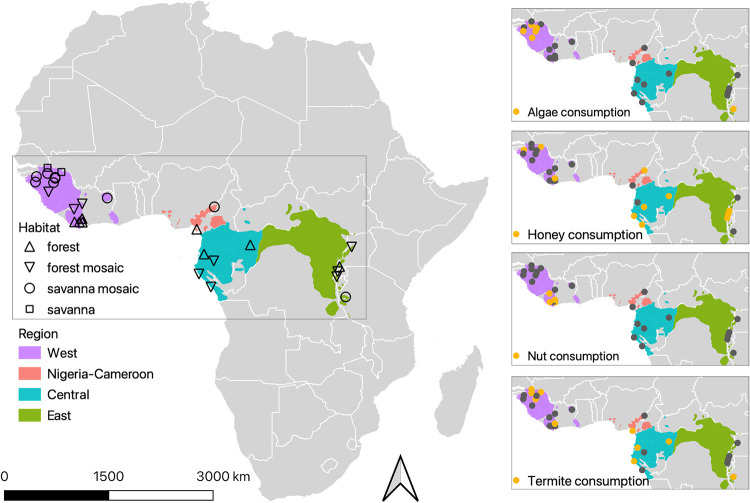
FIG 1.
Map (from https://open.africa/) of the 29 sites included in this study, showing either forest, forest mosaic, savanna mosaic, or savanna habitat types and the ranges of the four main geographic regions of chimpanzees (from reference 133). Insets show the variation among sites (points) in consumption of algae, honey, nuts (hard-shelled drupes), and termites (orange = consumed, gray = not consumed). In many cases, these items are accessed using tools (algae [6 of 7 sites], honey [10 of 13 sites], nuts [5 of 5 sites], termites [9 of 12 sites]).
Until very recently, the idea of comparing the microbiome of chimpanzees across populations spanning the full extent of their extant geographic range was unfeasible. Recently, however, the “Pan African Programme: The Cultured Chimpanzee” (PanAf; http://panafrican.eva.mpg.de/) has studied the behavioral diversity of chimpanzees across Africa (39) and sampled chimpanzee feces across that same geographic range (40). Building on these data and samples, it is possible to disentangle the relative influence of host genetics (and evolutionary history), climate, vegetation composition, and tool use on chimpanzee microbiomes, including both prokaryotes (bacteria and archaea) as well as eukaryotic parasites (including protist and nematode parasites and note that we use a lenient definition of parasites to include organisms that may not be harmful to the host or were originally listed as parasites but are now known to operate on a spectrum). Lastly, as chimpanzees are among the most closely related extant species to humans and comparisons are useful for understanding the evolution of the human gut microbiome (41), we compared the effects of geography, climate, and sex on the gut microbiomes of humans and chimpanzees. We hypothesized the following. (i) Large-scale geographic, genetic, and habitat differences among sites would drive differences in gut microbial community structure at the continental scale. (ii) Within regions, differences in diet due to vegetation composition and tool use would cause divergence in gut microbial community structure even within genetically similar populations owing to environmental and potential cultural influences on diet. (iii) Variation in the composition of gut parasite communities would mirror the observed variation in the prokaryotic communities due to similar factors influencing both components of the gut microbiome.
RESULTS
DNA was extracted from 560 fecal samples (originating from 560 different individuals) collected across the chimpanzee geographic range (Fig. 1), and we used 16S and 18S rRNA marker gene sequence data to characterize the prokaryotic (bacteria and archaea) communities and eukaryotic (parasite) communities, respectively, in each fecal sample. Permutational multivariate analysis of variance (PERMANOVA) and Mantel tests were conducted on pairwise Bray-Curtis dissimilarities (prokaryotes) and Jaccard dissimilarities (parasites) to test for the effects of genetics, geography, climate, habitat, diet, and sex (see Materials and Methods).
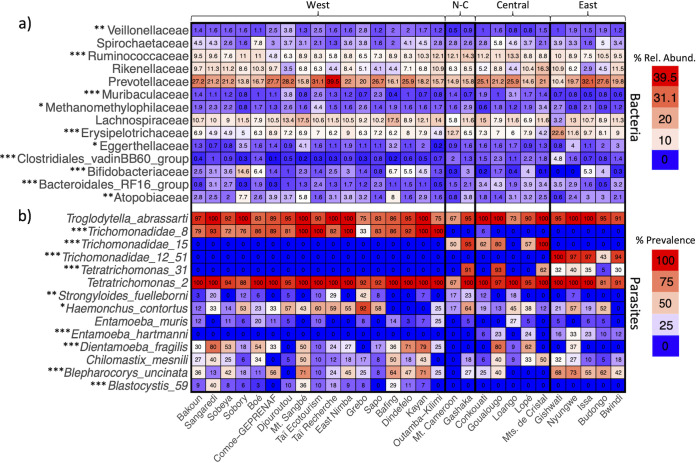
There were 14 bacterial families with mean relative abundances (number of reads out of 8,000 reads per sample) greater than 1% in at least one site. Among these, 10 families had significantly different relative abundances among the four regions (Kruskal-Wallis, Bonferroni, P < 0.05, Fig. 2a). The most abundant bacterial family was Prevotellaceae (mean ± standard error [SE] percent abundance, 22.1 ± 0.5), followed by Lachnospiraceae (10.5 ± 0.3), Ruminococcaceae (9.3 ± 0.2), Erysipelotrichaceae (7.9 ± 0.2), and Rikenellaceae (7.9 ± 0.3), all of which are the typical dominant taxa seen in other studies of chimpanzees, humans, and other primates (18, 42–44). None of the most common taxa were likely to be associated with soil, leaves, or other potential field contaminants (45, 46). Among the 14 parasite taxa examined here, 10 had significantly different occurrence probabilities among regions (logistic regression, P < 0.05, Fig. 2b). The two most prevalent parasites across the whole data set, and the only two parasites present in all sites, were identified as Troglodytella abrassarti and a Tetratrichomonas amplicon sequence variant (ASV) most closely related to Tetratrichomonas buttreyi. For the other parasites, there were large differences in prevalence among the regions, with some regions having no occurrences of a particular parasite and other regions having almost 100% prevalence.
FIG 2.
Heatmaps showing site mean percent relative abundances for 14 bacterial families with >1% mean relative abundance in at least one site (a) and parasite percent prevalence at each site (number of samples with parasite/total samples at site × 100) (b). Each panel is sorted from top to bottom in order of abundance (a) or prevalence (b). Note that there were 225 prokaryote families in the data set and if they were all included, columns would sum to 100. Different numbers after genera or families denote different ASVs; Trichomonadidae_12_51 represents two highly correlated ASVs that were combined. For more information about parasite taxonomy, see Table S3 at https://doi.org/10.6084/m9.figshare.14390426. Asterisks in panel a denote taxa with significantly different mean relative abundances among regions (Kruskal-Wallis, Bonferroni, *, P < 0.05; **, P < 0.01; ***, P < 0.001), and asterisks in panel b indicate taxa with significantly different probabilities of occurrence among regions (logistic regression, *, P < 0.05; **, P < 0.01; ***, P < 0.001). N-C, Nigeria-Cameroon.
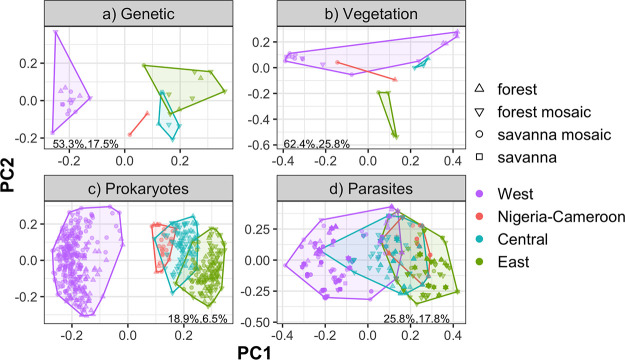
Geographic distance was significantly correlated with genetic, climate, and vegetation distance across the whole data set and within some, but not all, regions (Mantel test, P = 0.001, see Fig. S1 at https://doi.org/10.6084/m9.figshare.14607732). Geographic regions contained significantly different genetic populations of chimpanzees (PERMANOVA, pseudo-F = 15.6, R2 = 0.63, P = 0.001, Fig. 3a) as has been described elsewhere (47). Vegetation composition varied significantly among regions (PERMANOVA, F = 2.3, R2 = 0.23, P = 0.001, Fig. 3b). Regions also contained significantly different climates (PERMANOVA, pseudo-F = 4.5, R2 = 0.35, P = 0.001, see Fig. S2a at https://doi.org/10.6084/m9.figshare.14607735). Visual principal coordinate analysis (PCoA) grouping of samples by both vegetation composition and climate did not match the host genetic clustering, with the Nigeria-Cameroon, Central, and East regions grouped genetically (Fig. 3a), and the Nigeria-Cameroon, Central, and West regions grouped by vegetation (Fig. 3b) and climate (Fig. S2a at the figshare URL above).
FIG 3.
Principal coordinate analysis of chimpanzee site-level genetic distance [F′ST/(1 − F′ST)] (a), site-level vegetation distance (Bray-Curtis) (b), prokaryote (bacteria and archaea) community dissimilarity (Bray-Curtis) (c), and ubiquitous parasite community dissimilarity (Jaccard) (d). Numbers in the bottom corners represent the percent variation explained by principal coordinate 1 (PC1) and PC2, respectively. Point colors and convex hulls delineate the four geographic regions. n = 32, 27, 560, and 560 for a, b, c, and d, respectively. Overall, much of the structure in microbiomes is accounted for by genetic distance; note that the clustering in panel c matches the clustering in panel a rather than panel b. To see climate distances by region, see Fig. S2 at https://doi.org/10.6084/m9.figshare.14607735.
Variation in gut microbiota among regions.
Prokaryotic community composition differed significantly among the four geographic regions (Table 1 and Fig. 3c), with differences in the fecal microbial communities well correlated with site-level and individual-level genetic differences (Fig. 3a and c; see also Fig. S3 at https://doi.org/10.6084/m9.figshare.14607738). Parasite community composition also varied significantly among the four geographic regions (Table 1 and Fig. 3d). For both prokaryotes and parasites, all pairwise comparisons between regions were significant (P < 0.05). Additionally, parasite and prokaryotic community dissimilarities were significantly correlated across the whole data set (Mantel test, P = 0.001, see Fig. S4 at https://doi.org/10.6084/m9.figshare.14607741); i.e., changes in the composition of the prokaryotic communities were strongly associated with changes in the parasite communities.
TABLE 1.
PERMANOVA and PERMDISP resultsa
| Data set | Model structure | Variable | df | Pseudo-F | R2 | P | Fb |
|---|---|---|---|---|---|---|---|
| Prokaryotes | No strata (dfresidual = 552) | Region | 3 | 60.6 | 0.25 | 0.001 | 10.4*** |
| Sex | 1 | 1.39 | 0.002 | 0.11 | 0.9 | ||
| Region × sex | 3 | 1.15 | 0.005 | 0.19 | NA | ||
| Strata = region (dfresidual = 502) | Habitat | 3 | 28.33 | 0.1 | 0.001 | 52.4*** | |
| Diet | 8 | 15.07 | 0.13 | 0.001 | 16.1*** | ||
| Site | 17 | 10.9 | 0.2 | 0.001 | 6.4*** | ||
| Sex | 1 | 1.42 | 0.002 | 0.02 | 0.9 | ||
| Site × sex | 28 | 0.98 | 0.03 | 0.68 | NA | ||
| Parasites | No strata (dfresidual = 552) | Region | 3 | 84.96 | 0.31 | 0.001 | 5.0** |
| Sex | 1 | 2.02 | 0.002 | 0.07 | 0.6 | ||
| Region × sex | 3 | 1.41 | 0.005 | 0.12 | NA | ||
| Strata = region (dfresidual = 502) | Habitat | 3 | 20.74 | 0.06 | 0.001 | 16.9*** | |
| Diet | 8 | 20.04 | 0.16 | 0.001 | 12.7*** | ||
| Site | 17 | 13.98 | 0.24 | 0.001 | 2.0** | ||
| Sex | 1 | 2.21 | 0.002 | 0.04 | 0.6 | ||
| Site × sex | 28 | 1.05 | 0.03 | 0.35 | NA | ||
PERMANOVA and PERMDISP results showing degrees of freedom, pseudo-F, R2, and P values for PERMANOVA and F values with significance levels for PERMDISP for prokaryotes and parasites. For each data set, two models were run. The first model tested for the effect of region, sex, and their interaction, and the second model, stratified by region because of the large effect of region found in the first model, tested for effects of habitat, diet (consumption of algae, honey, nuts, termites, which typically require tools to access, see Table S1 at https://doi.org/10.6084/m9.figshare.14607687), site, sex, and a site-sex interaction. Note that the order of variable input matters but causes only minor changes in R2 values. For prokaryotes, if the order of habitat and diet is switched, habitat R2 = 0.08 and diet R2 = 0.15 (a change of 0.02). For parasites, if the order of habitat and diet is switched, habitat R2 = 0.08 and diet R2 = 0.15 (a change of 0.02 and 0.01).
F values with significance levels for PERMDISP for prokaryotes and parasites are shown as follows: **, P < 0.01, ***, P < 0.001. NA, not available.
Across the whole data set, geographic, genetic, climate, and vegetation distances were all significantly correlated with prokaryotic community dissimilarity and parasite community dissimilarity (Mantel test, P = 0.001, Fig. 4; see also Fig. S2 [https://doi.org/10.6084/m9.figshare.14607735], Fig. S3 [https://doi.org/10.6084/m9.figshare.14607738], and Fig. S5 [https://doi.org/10.6084/m9.figshare.14607744]). The generalized dissimilarity model (GDM) with geographic and vegetation predictor matrices indicated that geographic distance explained the most variation in prokaryote and parasite communities across the whole data set (Table 2). For prokaryotes, partial Mantel tests of vegetation while controlling for geographic distance were significant (r = 0.27, P = 0.001). In other words, once we accounted for the conjoined effects of geography and genetics, vegetation still had a significant influence on prokaryotic community composition. For parasites, partial Mantel tests of vegetation while controlling for geography were also significant (r = 0.08, P = 0.001).
FIG 4.
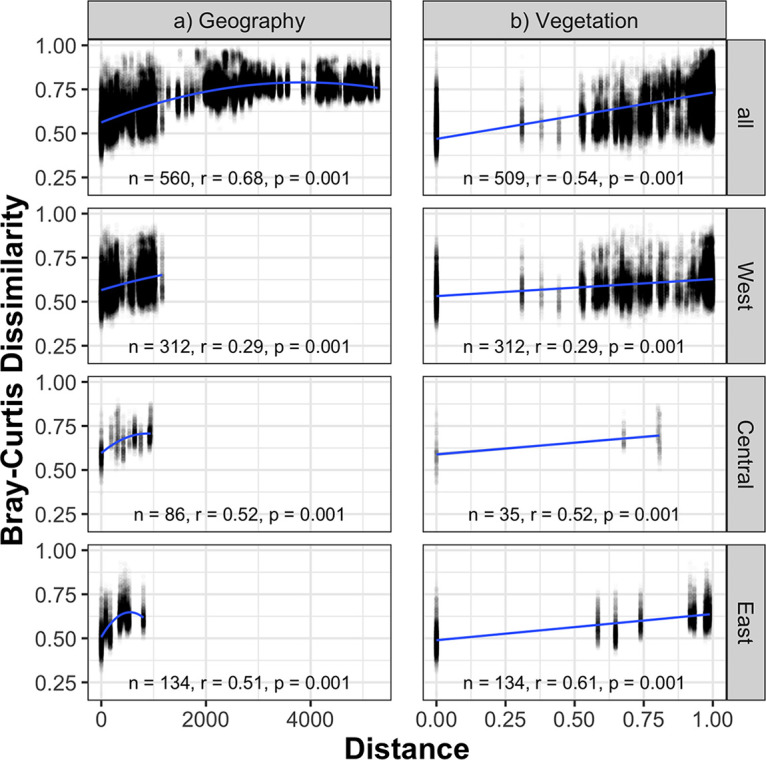
Prokaryote Bray-Curtis dissimilarity as a function of geographic distance (in kilometers), and vegetation dissimilarity (Bray-Curtis) for the whole data set and within the West, Central, and East regions. Within-region analyses for Nigeria-Cameroon are not shown here because there are only two sites. Statistics are from Mantel tests with Pearson r values. To aid in visualization, quadratic (geography) and linear (vegetation) models are displayed. To view similar figures in relation to climate distance, rather than geographic distance, see Fig. S2 at https://doi.org/10.6084/m9.figshare.14607735. To view similar figures in relation to genetic distance, see Fig. S3 at https://doi.org/10.6084/m9.figshare.14607738.
TABLE 2.
Generalized dissimilarity modeling resultsa
| Data set | Predictor matrix | All regions | West region | Central region | East region |
|---|---|---|---|---|---|
| Prokaryotes | Geography | 0.697 | 0.097 | 0.133 | 0.000 |
| Vegetation | 0.067 | 0.174 | 0.213 | 0.344 | |
| % deviance explained | 56.29 | 9.75 | 26.67 | 37.99 | |
| Parasites | Geography | 0.606 | 0.057 | 0.000 | 0.180 |
| Vegetation | 0.000 | 0.111 | 0.265 | 0.000 | |
| % deviance explained | 22.54 | 1.29 | 8.22 | 1.58 | |
Generalized dissimilarity modeling results showing the maximum partial ecological distance explained by geographic distance and vegetation dissimilarity predictor matrices while controlling for the other, as well as the deviance explained by the model. Models were run with the whole data set as well as within the West, Central, and East regions. Boldface values are the most important predictors in each data set.
At the prokaryote ASV level, there were 45, 7, 15, and 15 indicator ASVs for the West, Nigeria-Cameroon, Central, and East regions, respectively; indicator taxa for a region are both more abundant and more common in that region (48). These indicator taxa were randomly distributed across the phylogenetic tree (see Fig. S6a at https://doi.org/10.6084/m9.figshare.14607750), highlighting that they are not restricted to particular prokaryotic lineages and contain both spore-forming and non-spore-forming taxa.
Variation in gut microbiota within regions.
To examine local effects and control for genetic differences, we also performed analyses within regions. Such analyses are also important to understand the extent to which broad patterns are generalizable or are contingent upon the strong differences observed across regions. Community dissimilarities of both prokaryotes and parasites were significantly greater in between-site sample comparisons of the entire data set than in within-site sample comparisons (Wilcoxon test, P < 0.001), and this pattern persisted in analyses within the four geographic regions (see Fig. S7 at https://doi.org/10.6084/m9.figshare.14607756). Within geographic regions, habitat type, diet (consumption of algae, honey, nuts, and termites, all of which are often consumed with tools), site identity, and to a lesser extent sex, affected prokaryotic and parasite community composition (Table 1). As with regional differences, there were strong differences in bacterial family abundances and parasite prevalence among sites in the same region (Fig. 2). Strikingly, certain parasite taxa had no occurrences in some sites and almost 100% prevalence at other sites within the same region (Fig. 2b).
Geographic, climate, and vegetation distances were all significantly correlated with prokaryotic community dissimilarity and parasite community dissimilarity within each region represented by more than two sites (i.e., three regions) (Mantel test, P = 0.001, Fig. 4; see also Fig. S2b [https://doi.org/10.6084/m9.figshare.14607735] and Fig. S5 [https://doi.org/10.6084/m9.figshare.14607744]). Individual-level genetic distances were significantly correlated with prokaryote community dissimilarity in all regions except for the West region and with parasite community dissimilarity in the Nigeria-Cameroon and Central regions (see Fig. S3 at http://doi.org/10.6084/m9.figshare.14607738). Additionally, parasite and prokaryotic community dissimilarity were significantly correlated within each region (Mantel test, P = 0.001, see Fig. S4 at http://doi.org/10.6084/m9.figshare.14607741). For prokaryotes, partial Mantel tests of vegetation while controlling for geography were significant within the East (r = 0.40, P = 0.001), Central (r = 0.29, P = 0.001), and West (r = 0.09, P = 0.001) regions. On the other hand, for parasites, partial Mantel tests of vegetation while controlling for geography were significant only in the Central (r = 0.32, P = 0.001) and West (r = 0.04, P = 0.03) regions. Full GDM models within each region indicate that the relative importance of vegetation was greater than geography, in contrast to the models built from the whole data set (Table 2).
Some site-specific differences were observed and were more strongly associated with prokaryote community composition than parasite community composition (Table 1; see also Fig. S8 at https://doi.org/10.6084/m9.figshare.14390369), although no such associations were found in the West region (subspecies verus). Knowing that some chimpanzee populations use tools to acquire certain specialty items in their diet, we categorized each site by the observed collection of specialty items they consume. For example, from long-term data, it is known that chimpanzees at Gashaka in the Nigerian-Cameroon range consume honey (49), but not termites (50). At Mount (Mt.) Cameroon, neither has been observed after 1 year of observation, and the possible difference in consumption of these items may contribute to the different microbial community composition between the two sites (see Fig. S8a and b at the figshare URL above). The five sites in the East region contained two different specialty diets, honey (Bwindi, Gishwati, and Nyungwe) and algae and termites (Issa), and a site (Budongo) where chimpanzees were never observed to consume any of those items (over 1 year of observations), although longer-term observations are needed as consumption of honey and termites was observed in a neighboring community of chimpanzees (51, 52); these three groupings were associated with distinct prokaryote communities (Fig. S8a at the figshare URL above). In the Central region, gut prokaryotic composition at the one site where only termite consumption was observed (Mts. de Cristal) was significantly different than at the two sites where only honey consumption was observed (Lopé and Conkouati, Fig. S8a at the figshare URL above). At the long-term field site of Goualougo, both termites and honey are known to be consumed (53), and at Loango, underground honey is consumed with tools (54), while termite consumption without tools has been observed. For consumption of honey and termites, which occurs in all four regions, there were 80 and 61 prokaryotic indicator taxa, respectively, distributed across the phylogenetic tree (see Fig. S6b at https://doi.org/10.6084/m9.figshare.14607750). Indicator taxa were shared among more than one region in only a few instances, likely reflective of the broader differences in community composition among the regions. Together, these results suggest that tool use may affect the composition of the gut microbiome by making certain food items available, but we recognize that tool use is difficult to disentangle from other factors that may also differ across chimpanzee populations, including differences in consumption of the various plant foods that make up the bulk of the chimpanzee diet or other prey items (16).
Comparison with humans.
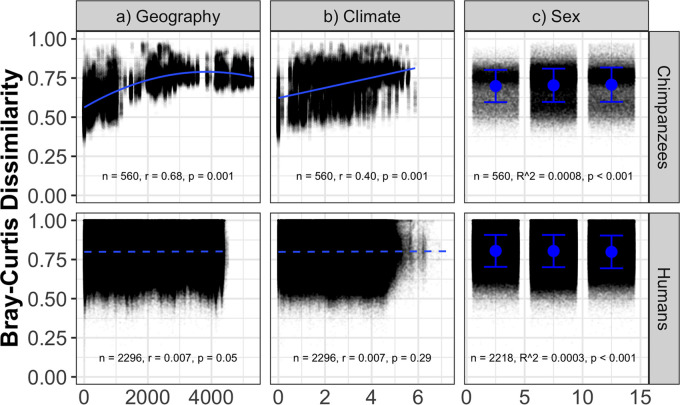
To contrast the effects of geography, climate, and sex on the gut microbiome in chimpanzees versus a population of industrialized humans known to have a relatively homogeneous diet and a high degree of interregional connectivity, we analyzed a comparable data set from humans enrolled in the American Gut Project (17) (see Materials and Methods). In contrast to the patterns observed for the chimpanzee gut microbiome, geographic distance and climate distance were not significantly correlated with human gut microbiome dissimilarity (Mantel test, P ≥ 0.05, Fig. 5a and b). While geography and climate had significant and strong (r = 0.66 and 0.44, respectively) effects on the chimpanzee gut microbiome, these same factors explained very little variation (r = 0.007) in the human gut microbiome. The effects of host sex were minor in both species (R2 = 0.0008 in chimpanzees, R2 = 0.0003 in humans, Fig. 5c).
FIG 5.
Relationship between geographic distance (in kilometers), climate distance (Euclidean distance), sex (FF = female-female comparisons, FM = female-male comparisons, MM = male-male comparisons), and prokaryote (bacteria and archaea) community composition in chimpanzees and humans. For chimpanzees, a quadratic (geography) and linear (climate) trendline is shown, while for humans a dashed, nonsignificant, linear trendline is shown. Blue points and error bars represent means and standard deviations, respectively. Statistics are from Mantel tests (geography and climate) and analysis of variance (ANOVA) (sex). Note that while there were significant effects of sex on dissimilarity, and male-male comparisons were more dissimilar than female-female and female-male comparisons in chimpanzees, but less dissimilar in humans, the extremely small effect sizes in both species suggests sex does not structure the gut microbiome in either species.
DISCUSSION
Variation in gut microbiota among regions.
Previous studies on the chimpanzee gut microbiome have been limited in both sample size and geography, with most studies sampling only one of the four subspecies (e.g., references 50, 55, and 56). Our comprehensive sampling scheme enabled us to examine geographic and genetic effects on the chimpanzee microbiome. To date, few studies of any taxon have considered the variation in microbiomes among individuals within a species at such a scale, except for humans (57–59). On the basis of studies of humans (17, 60, 61), we expected there to be a weak effect of geographic and genetic distance on chimpanzee gut microbiota. Instead, we found that much of the variation among chimpanzees’ gut microbiomes was associated with geographic distance and genetic distance. This pattern was observed for both prokaryotes and eukaryotic parasites, including both parasitic worms and protists.
The effects of geographic and genetic distance were apparent both among and within geographic regions. Such effects could be due to either neutral divergence (42, 62, 63), disease (64), or some effect of host genetic identity on microbiome composition. The simplest (and most neutral) model is one in which microbiomes are inherited by individuals from their mother during birth and subsequently acquired from community members. The two predictions of this model, that geographic and genetic distance, which are strongly correlated in chimpanzees, are strongly predictive of prokaryote community composition and more weakly predictive of parasite community composition, are both supported by our data. The weaker predictive power for parasites is possibly because many parasite taxa (especially macroparasites) are acquired from dietary sources and the environment, in addition to directly from other chimpanzees via fecal-oral transmission (65), but it could also be due to the much lower number of parasite taxa compared to bacteria. Thus, we suspect that the relationship between host genetic differences and microbiome genetic differences primarily reflects history and neutral divergence. The relationship between genetic relatedness and gut microbiota observed at the continental scale in our data has not been previously documented, as previous work has focused on individual chimpanzee communities. Within individual chimpanzee communities, gut microbiome composition does not relate to genetic relatedness (e.g., parents and children and siblings) (66) but is rather influenced by social contact (67), with similar patterns also observed in baboons (5, 68), black howler monkeys (69), and sifakas (70). We cannot exclude the possibility of associations between specific host genes and microbiome composition, but we could not identify such associations here as the microsatellite genes provide information only on host genetic divergence.
Alternatively, some of the observed effects of geography and host genetics may be attributed to climate and vegetation, which were both correlated with geography (see Fig. S2 at https://doi.org/10.6084/m9.figshare.14607735), as well as tool use for special dietary items (e.g., hard-shell drupes are eaten only in part of the West region, see Table S1 at https://doi.org/10.6084/m9.figshare.14607687). Climate is unlikely to directly impact gut prokaryotes; many gut-associated prokaryotes cannot proliferate outside the gut. However, climate could affect eukaryotic members of the gut via effects on the presence and identity of alternate hosts at a particular site (65). In addition, climate can indirectly affect both prokaryotes and eukaryotes by influencing the vegetation composition and, consequently, the foods available at a particular site (71). Here, we find that across the entire chimpanzee range, the effects of climate, habitat type, and vegetation composition on the gut microbiome are modest, suggesting that the broad-scale geographic patterns we observed are only partially explained by climate and habitat.
In contrast to the pattern we observed for chimpanzees across sub-Saharan Africa, we found no geographic structure in the microbiomes of humans living across a similar geographic extent, the contiguous United States. Based on an analysis of 2296 samples, the microbiomes of humans in the contiguous United States are not correlated with geography or climate. For example, people in the United States living in New York and California can have as similar gut microbiota as two New Yorkers despite living in different climates 4,000 km apart. While other studies have shown differences in the human gut microbiome based on geography (e.g., reference 72), those studies reflect the effects of diet and lifestyle rather than geographic distance (18). Assuming that the patterns in gut microbiomes of chimpanzees are reflective of those of our last common ancestor, this lack of pattern in human microbiome data, at least in an industrialized Western society, suggests that humans have obscured ancient patterns in microbiome biogeography since diverging from chimpanzees, as has been observed with human effects on other ecosystems (56). This is likely due to a variety of processes associated with industrialization and globalization including, but not limited to, the decoupling of food and climate, and increased cross-regional traveling, movement, and social contact.
Variation in gut microbiota within regions.
In addition to the broad regional differences we observed in chimpanzee microbiomes, there were also strong site-level differences within regions, which is likely amplified by higher rates of social contact among chimpanzees living in the same site (5, 67). Variation among sites within regions was correlated with geographic distance and genetic distance (just as with the among-region comparisons) but also with vegetation and consumption of specific diet items (16). It has been noted previously that even neighboring chimpanzee communities can have very different gut microbiomes (66). Here, we suggest that while some of the site differences are environmentally driven, the socially mediated use of tools may also possibly contribute to gut microbiome variation via diet.
Chimpanzees are one of the few species that employ the socially mediated use of tools, arguably a component of their foraging cultures (29, 31, 34, 38, 73), which influences which foods they eat and which tools they use to eat those foods. Consequently, differences between the foods available in a particular region and the foods actually consumed in a region may vary. The effect we observe of the consumption of algae, honey, nuts, and termites, which are consumed with the help of tools in many cases, suggests the potential influence of tool use on the gut microbiome via diet (44, 74).
Additional work is required to identify potential mechanisms of any associations between tool use and diet on microbiomes and the potential implications for host health. Here, we focused on four dietary items observed to be accessed using tools, although only one of them—nuts—is exclusively consumed when tools are used. Consumption of these four dietary items was possibly associated with differences in microbiome composition among sites in some instances. Since most of these items are eaten in large quantities, at least seasonally, it is possible that they offer different nutritional profiles that could select for different prokaryotic taxa in the gut. Algae, which are consumed at an average rate of ∼360 g/day during some months, are an important source of nutrients for chimpanzees (30) and feature recalcitrant complex carbohydrates; in fact, the gut bacteria of some human populations appear to have acquired the ability to produce specialized enzymes to digest seaweeds (75). Alternatively, honey provides high concentrations of simple sugars (76). In humans, sugar-rich diets are associated with increases in Bifidobacteria and decreases in Bacteroides (77); we observed one Bifidobacteria ASV associated with honey consumption in the Nigeria-Cameroon region, but this comparison should be made cautiously, as chimpanzee honey consumption is lower than human sugar consumption. The nuts such as oil-palm (Elaeis guineensis) and Coula edulis consumed by chimpanzees in the West region are rich in fat (78) and are consumed in large quantities in some months (700 g/day, net gain of 3,450 kcal/day) (72). Lastly, termites are sources of protein, iron, and manganese and are a valuable part of chimpanzee nutrition (79). However, we do not have comprehensive data on everything consumed by the chimpanzees at each site, and thus, there remain potential alternative explanations for site differences. For example, the three sites in the Central region where both honey and termites are consumed (Mts. de Cristal, Goualougo, and Loango) had distinct microbiomes, possibly due to the consumption of unique prey species, such as tortoises at Loango (80), or consumption of different fruit species. Furthermore, any differences between the Taï sites and other sites in the West region could be partially due to hunting of red colobus monkeys (16). While we do not have data on the frequency of tool use or the quantity of consumption for all of these specific diet items, we speculate that consumption of these items, if it is indeed contributing to these patterns, must be frequent and long term, as has been shown in certain sites (30, 76, 78, 79). For example, long-term dietary patterns in humans have far larger effects on the gut microbiome than short-term changes in diet (81). Continued long-term observations as well as DNA examination of feces for plants, arthropods, and other dietary items will provide additional insights into the relationships between diet and microbiome composition.
Parasites.
There is a long history of research on parasites in primates (65), including many studies focused on chimpanzees (e.g., references 51 and 82). Until recently, however, parasite surveys used morphology-based identification, and newer sequencing studies have been limited in their geographic breadth or sample size (83–86). As a result, while we have a rich understanding of the presence of certain parasites in certain chimpanzee populations (65), our understanding of the distribution of parasites lags behind that of bacteria (85, 87). Our survey of gut microbial eukaryotic parasites sheds new light on parasite distributions in chimpanzees and the factors influencing those distributions. Only two parasites—one Tetratrichomonas ASV and the ciliate Troglodytella abrassarti—were present in all sites. Troglodytella abrassarti was not only found in all chimpanzee sites and in 94% of samples, but is also known to be common in other primates (88). This ciliate appears to be an important and beneficial component of the gut microbiome, contributing to polysaccharide hydrolytic activities in the chimpanzee colon (89). In contrast, most parasite species had more restricted patterns of distribution. Such between-site differences may be amplified by minimal social contact between sites, which is restricted to some immigration, while prevalence may be high within sites due to the social contact among individuals living at a site (87). In other primates, social contact has been observed to affect transmission of the nematode Strongyloides (82), but not protists (90, 91). Most parasites were also not consistently found in individuals with multiple samples, suggesting that they are transient in the gut and/or sourced from the environment or diet, or difficult to detect with 18S rRNA gene data (see Fig. S9 at https://doi.org/10.6084/m9.figshare.14390372).
As with the gut prokaryotes, dissimilarity in parasite communities was correlated with geography, climate, and vegetation, although the relationships for parasites were weaker than for prokaryotes, as has been found in other studies (85). Previous work has found more closely related primates to have more similar parasite communities (86), an observation we extend to the subspecies and population level. For example, parasites in the parabasalid Tetratrichomonas genus are prevalent in sympatric chimpanzees, gorillas, and humans, but different species and strains are found among the three species (84); different lineages within the genus are associated with different hosts, with a high degree of specificity (92). We report host specificity, at the regional level, in four of five trichomonads. Chimpanzees that eat insects as well as plants are expected to acquire some of the parasites present in the insects (65, 93), which likely contributed to the relationship between diet (e.g., termite consumption) and parasite community composition. Furthermore, the four items studied here contribute to chimpanzee nutrition, which may also influence parasite loads, as has been found in deer (94), bovids (95), and rats (96).
Dissimilarity among sites in prokaryotic communities and parasite communities was significantly correlated, which may be due to the fact that both prokaryotic communities and parasites are similarly influenced, both directly and indirectly, by geography, climate, and vegetation. It is also possible that the presence or absence of certain parasites can influence bacterial community composition or that the bacterial community influences parasite establishment. Many such transdomain correlations have been observed previously in both humans and chimpanzees (83, 97). In humans, the well-studied protozoan parasite Blastocystis is associated with lower abundances of Bacteroides bacteria (98), while the presence of Entamoeba can be predicted with 79% accuracy based on gut bacterial community composition (97). In chimpanzees, Blastocystis and Strongyloides carriers were associated with different bacterial communities (83). Several mechanisms of parasite-bacterium interactions have been proposed in the vertebrate gut (99). For example, parasites, including helminths and Entamoeba, can stimulate mucus production and alter mucosal composition, which may in turn affect nutrient availability and movement and attachment sites in the gut. Microbial eukaryotes can also play roles in nutrient cycling and bacterial turnover, and some, including Entamoeba, directly feed on bacteria (98, 100).
Many of the most prevalent parasites observed in our data set are common in humans and other mammals (88) and are associated with host health. Dientamoeba, Blastocystis, and Entamoeba are very common in humans and have been found to be more prevalent in healthy humans relative to humans with irritable bowel syndrome, inflammatory bowel disease, or other gastrointestinal disorders (98). Thus, these parasites may be potentially beneficial to the host under certain conditions (101). Alternatively, their prevalence may simply be a function of higher intestinal oxygen concentrations in humans with intestinal dysbiosis-linked diseases (98). In most cases, gut microbial eukaryotes move along the parasitism-mutualism spectrum in a context-dependent manner which may also be modulated by gut bacteria (99, 102).
Conclusions.
Our geographic range-wide survey of the chimpanzee gut microbiome, including both prokaryotes and eukaryotic parasites, combining data on chimpanzee genetics, geography, climate, vegetation, and tool use to acquire specific foods, partially disentangles the drivers of gut microbiota composition across scales and synthesizes results from a number of previous local studies. The use of tool behavioral data in chimpanzees to explain differences in gut microbiota is a novel approach which may shed light on how diet, and the potential cultural behaviors that affect diet, can shape the gut microbiome and may have played a role in the evolution of the human microbiome. While isolation, either through genetic differences or geographic separation, outweighs climate, vegetation, and diet factors at large scales, climate, vegetation, and tool use all are correlated with gut microbiome composition at local scales, likely due to changes in diet. Geographic distance and climate played comparatively stronger roles in structuring the chimpanzee gut microbiome than the industrialized human gut microbiome, a product of technologies and developments that have decoupled humans from their local environments and food sources.
MATERIALS AND METHODS
Experimental design.
We collected feces from 29 different sites from a total of 14 countries spanning four main geographic regions of chimpanzee (Pan troglodytes) populations across the African continent (Fig. 1). To compile such a comprehensive sample set, samples were collected from multiple years and in all 12 months of the year, but year and season (wet/dry) had minimal effects on community composition (PERMANOVA R2 = 0.02 and 0.01, respectively, for prokaryotes and 0.02 and 0.02, respectively, for parasites). Regions are defined as West, Nigeria-Cameroon, Central, and East, which correspond to the Pan troglodytes verus, ellioti, troglodytes, and schweinfurthii subspecies, respectively. At each site, we aimed to collect feces from a single chimpanzee community, but because some of the groups are not habituated to human presence and therefore not observed directly, it is possible that samples from neighboring communities were inadvertently collected at a given site. Of the 560 samples used in the microbiome analysis, the number of samples per site ranged from 4 to 38 (see Table S2 at https://doi.org/10.6084/m9.figshare.14390420). Annual temperature, temperature seasonality (standard deviation × 100), annual precipitation, and precipitation seasonality (coefficient of variation) were extracted for each site from the BioClim data set (https://gitlab.com/tpoisot/BioClim/tree/master/assets). Vegetation composition data were collected according to the PanAf protocol (103) except for five sites which are described in Appendix 1 at https://doi.org/10.6084/m9.figshare.14390378. To summarize, at each site, transects spaced 1 km apart were established. Along these transects, 20 m × 20 m habitat plots were placed generally every 100 m (but up to 1,000 m); additional habitat plots were surveyed along gallery forest in sites with dominant savanna vegetation. The surveyed area ranged from 3 ha to 24 ha depending on the size of the site. Within each plot, all trees, lianas, and shrubs with a diameter at breast height (DBH) of ≥10 cm were taxonomically identified, and their diameter was measured at 1.3-m height. Site habitat type was defined broadly as either forest (mean ± SE plant species richness, 100 ± 11), forest mosaic (98 ± 21), savanna mosaic (78 ± 10), or savanna (58 ± 0) following previous work (104). Data on tool use behaviors to acquire algae, honey, nuts (technically hard-shell drupes), and termites come from the following sources: (i) extensive camera trap footage; (ii) fecal samples that provided evidence of ingestion of insects, algae, and honey, resources often exploited with the aid of tools; and (iii) evidence of tool use identified during reconnaissance, line, and strip transect surveys (39, 105) (see Table S1 at https://doi.org/10.6084/m9.figshare.14607687). We selected these four food acquisition behaviors because they generally occur at fixed locations with artifacts and are therefore relatively easy to detect via camera traps, exhibit variation across communities rather than being universal traits of chimpanzees, and target unique dietary items that could potentially affect the gut microbiome even if the majority of the chimpanzee diet is fruit. There is evidence of tool use to eat algae (6 of 7 sites where algae is consumed), honey (10 of 13 sites), nuts (5 of 5 sites), and termites (9 of 12 sites); for the remaining sites, we do not know whether tools for these dietary items were used or not, only that it has not been documented thus far. We did not include analysis of data on the consumption of other items such as meat and bone marrow as it is beyond the scope of the current observational record to reliably distinguish presence versus true absence at a site. Thus, we recognize that we account for only a fraction of the potential dietary differences in chimpanzees.
Feces collection, extraction, and individual identification.
Chimpanzee fecal samples were collected throughout the year and preserved according to the two-step ethanol-silica method (106), stored in the field for up to 2 years, and then stored in the lab at −20°C prior to extraction. Feces were up to 3 days old as determined by field staff. The effects of time between defecation and sampling have been shown to be minor (7). Due to the presence of dung beetles, rain, and maggots, ape feces generally do not persist for more than a few days (107). Furthermore, samples older than 3 days are highly degraded and generally do not yield viable DNA for reliable chimpanzee genotyping (108). DNA was extracted from the samples using the QIAamp 96 PowerFecal QIAcube HT robot and kit (Qiagen, Hilden, Germany) with a pretreatment step to improve DNA yield (40). Microsatellite genotypes were obtained for up to 15 microsatellite loci using a two-step multiplex PCR method (109) with slight modifications (40) and electrophoresing the amplicons using an ABI PRISM 3130 Genetic Analyser and GeneMapper v 3.7 software (Applied Biosystems, Foster City, CA, USA) to visualize and score the results manually. Individual identity was assigned based on matching genotypes at a minimum of seven loci (110) from at least three PCR replicates for homozygotes and at least two replicates for heterozygotes (40). A fixation index (Fst) genetic distance matrix at the site level was calculated from these genetic data. Additionally, an individual-level genetic distance matrix was calculated using the Smouse and Peakall metric (111) with the GenoDive software program (112).
Microbiome analysis.
Extracted DNA was transported on dry ice to the Fierer lab at the University of Colorado Boulder for PCR amplification and sequencing using the primers and methods of the Earth Microbiome Project (113). For prokaryotes (bacteria and archaea), we targeted the V4 region of the 16S rRNA gene using the 515F/806R primer pair, modified to include the necessary Illumina adapters. For eukaryotes, we targeted the V9 region of the 18S rRNA gene using the 1391f/EukBr primer pair. Following PCR, DNA was pooled, normalized with the SequalPrep normalization plate kit (Invitrogen, Carlsbad, CA, USA), and then sequenced on the Illumina MiSeq platform using 2 × 150 bp chemistry at the BioFrontiers Institute (Boulder, CO, USA). Amplicon reads were demultiplexed using the open source “idemp” tool (https://github.com/yhwu/idemp), and adapters were cut from the sequences using the open source “cutadapt” tool (114) (https://cutadapt.readthedocs.io/en/stable/) with default parameters and --minimum-length set at 50. Sequences were then quality filtered (16S parameters maxEE = 1, truncQ = 11, maxN = 0; 18S parameters maxEE = 2, truncQ = 2, maxN = 0), trimmed (150 and 145 bp for 16S, 103 bp for 18S) and merged (only 16S) using the DADA2 pipeline (115) to then infer amplicon sequence variants (ASVs) (116) and remove chimeras. 18S rRNA gene reads were not merged, and only the forward reads were used due to the variable length of the amplified region. Using the DADA2 pipeline, taxonomy was assigned using the SILVA database (117) version 132 (https://www.arb-silva.de/) for 16S rRNA sequences and the PR2 database (118) (https://pr2-database.org/) for 18S rRNA sequences. A phylogenetic tree of the 278 most abundant (>0.0625% mean relative abundance) and ubiquitous (present in >5% of samples) bacteria and archaea was constructed by using PyNAST (119) to align sequences and FastTree (120) to construct the tree using the QIIME program (121). Trees were visualized using the ggtree (122) R package. Eukaryote, chloroplast, and mitochondrial sequences were removed from the 16S rRNA sequence data set, as well as any ASV not assigned to either the bacterial or archaeal domains. 16S rRNA gene sequence data were then rarefied to 8,000 sequences per sample. Taxonomic filtering and rarefaction were performed using the mctoolsr (123) R package. This sequencing depth is adequate to capture most of the richness of ASVs in each sample, which ranged from an average of 196 to 247 ASVs per sample depending on the region (see Fig. S10 at https://doi.org/10.6084/m9.figshare.14390375). 18S rRNA gene sequence data were not rarefied, but samples with less than 3,100 sequences per sample before any taxonomic filtering were removed (samples should also have abundant plant and chimpanzee DNA). We identified 11 different ASVs that were present in at least 10% of samples, all of which were from known parasite species based on the literature (134). We took a conservative approach (to avoid false-positive results) and defined presence as having ≥50 sequences in a sample. To avoid counting two ASVs that likely represent the same parasite taxon as separate taxa, we ran correlations on read abundances and used BLAST analysis of ASV sequences for any ASVs identified as belonging to the same family, combining those ASVs that were strongly correlated and identified as belonging to the same species. Two of the ASVs that were significantly and strongly correlated (r = 0.82, P < 0.001) and closely related (percent identity = 98.06) were combined. Furthermore, due to previous work (83, 124) on known chimpanzee parasites from the Blastocystis, Strongyloides, and Entamoeba genera, we included a Blastocystis ASV, a Strongyloides ASV, and two Entamoeba ASVs that were present in at least 5% of samples, for a total of 14 parasite ASVs (see Table S3 at https://doi.org/10.6084/m9.figshare.14390426). These most prevalent and known chimpanzee parasites are unlikely to be sourced from prey species (93).
Statistical analysis.
A total of 852 fecal samples passed the rarefaction cutoff of 8,000 16S rRNA gene reads per sample. These samples originated from 577 unique individuals (based on the microsatellite analysis described above), 147 of which were sampled multiple times, and 17 of which did not have sex identification. Within the same site, prokaryotic community Bray-Curtis dissimilarity was significantly greater between individual chimpanzees than within the same individuals that were sampled multiple times (see Fig. S11 at https://doi.org/10.6084/m9.figshare.14390381). To avoid statistical issues due to repeated sampling, we included only one sample per individual (using the first sample of each individual) in the analysis. Thus, we focused analyses on 560 fecal samples that originated from unique individuals and for which sex was identified (257 female, 303 male). To assess differences in community composition, we calculated Bray-Curtis dissimilarity for the prokaryote data and Jaccard dissimilarity for parasites. We conducted Wilcoxon tests to analyze whether Bray-Curtis or Jaccard dissimilarities were significantly different within versus among sampling sites, both on the entire data set and within each geographic region. We conducted principal coordinates analysis to visualize differences among samples with regard to genetic distance, climate distance, vegetation distance, prokaryote community composition, and parasite community composition. We used permutational multivariate analysis of variance (125) (PERMANOVA) implemented in the vegan (126) R package to assess the effects of geographic region on genetic, climate, and vegetation distance and the effects of geographic region, habitat type, diet, site identity, and sex on prokaryotic and parasite community composition. Pairwise PERMANOVAs were performed with the RVAideMemoire (127) R package. Multivariate homogeneity of group dispersions was tested with permutational analysis of multivariate dispersions (PERMDISP) implemented in vegan. We used Kruskal-Wallis tests with Bonferroni P value correction to analyze differences in the mean relative abundances of top bacterial families among the four geographic regions and logistic regressions to analyze differences in parasite occurrence probabilities among the regions. We used multilevel pattern analysis implemented in the indicspecies (48) R package to identify indicator taxa for each geographic region as well as for the presence and absence of consumption of honey and termites, which are consumed in all four regions (algae are consumed in two regions and nuts in one region).
To analyze the effects of geographic distance, climate, and vegetation on prokaryote and parasite community composition, we used Mantel tests and partial Mantel tests (controlling for geographic distance) between distance/dissimilarity matrices. Additionally, to further assess the relative importance of these predictor matrices, we used generalized dissimilarity modeling (128) (GDM) implemented in the gdm (129) R package. These analyses were performed on the entire data set as well as separately within the West, Central, and East regions; within-region analyses were not performed for Nigeria-Cameroon as there were only two sampling sites in this region. Climate distance was calculated as the Euclidean distance of annual mean temperature, annual mean precipitation, temperature seasonality, and precipitation seasonality, which were scaled (to 0 mean unit variance) before the calculation. Vegetation “distance” was calculated as Bray-Curtis dissimilarity in plant species composition.
To contrast the effects of geography, climate, and sex on the gut microbiome in chimpanzees versus in Westernized humans, we conducted Mantel tests between geographic distance, climate distance, and prokaryotic community Bray-Curtis dissimilarity for 2,296 human gut samples from the American Gut Project (17). This data set has readily and publicly available ASV tables, a similar geographic extent and sample size as our study, and used the same primers as our study. As previous studies have already investigated differences between the microbiomes of nonhuman primates and humans in different locations and societies (19, 42, 63, 130, 131), the goal here was simply to contrast the effects of geographic distance, climate, and sex in chimpanzees with a population of industrialized humans known to have a relatively homogeneous diet and a high degree of interregional connectivity. The effect of sex was assessed by comparing Bray-Curtis dissimilarities among female-female, female-male, and male-male pairwise comparisons. The human samples are from adults in the contiguous United States who did not take antibiotics in the year prior to sampling. Samples were rarefied at 10,000 sequences per sample and processed using Deblur (132) as outlined in reference 17. Data are publicly available and were downloaded from ftp://ftp.microbio.me/AmericanGut/manuscript-package/10000/distance/ on 24 March 2020.
Data availability.
Raw sequencing data and ASV sequences were deposited to NCBI SRA and GenBank, respectively, under the BioProject accession number PRJNA625726.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Max Planck Society, the Max Planck Society Innovation Fund, and the Heinz L. Krekeler Foundation for funding. J.F.G. was supported by the Deutsche Forschungsgemeinschaft (DFG) Research Group “Sociality and Health in Primates” (FOR2136). Publication of this article was funded by the University of Colorado Boulder Libraries Open Access Fund.
We thank Adeline Fayolle and Julie Aleman for sharing their vegetation GIS layers with us for classifying chimpanzee habitats. We thank the following authorities and agencies who kindly granted permission to conduct research: Agence Nationale des Parcs Nationaux, Gabon; Centre National de la Recherche Scientifique (CENAREST), Gabon; Société Équatoriale d’Exploitation Forestière (SEEF), Gabon; Conservation Society of Mbe Mountains (CAMM), Nigeria; National Park Service, Nigeria; Department of Wildlife and Range Management, Ghana; Forestry Commission, Ghana; Direction des Eaux, Forêts et Chasses, Sénégal; Eaux et Forêts, Mali; Ministre de l'Environnement et de l'Assainissement et du Développement Durable du Mali, Mali; Forestry Development Authority, Liberia; Institut Congolais pour la Conservation de la Nature, Democratic Republic of Congo (DR-Congo); Ministère de la Recherche Scientifique, DR-Congo; Ministère de l’Économie Forestière, Republic of Congo (R-Congo); Ministère de la Recherche Scientifique et Technologique, R-Congo; Agence Congolaise de la Faune et des Aires Protégées, R-Congo; Ministère de la Recherche Scientifique et de l'Innovation, Cameroon; Ministère des Forêts et de la Faune, Cameroon; Ministère de l'Agriculture de l'Élevage et des Eaux et Forêts, Guinée; Ministère des Eaux et Forêts, Ivory Coast, Office Ivoirien des Parcs et Réserves, Ivory Coast; Ministro da Agricultura e Desenvolvimento Rural, Guinea-Bissau; Instituto da Biodiversidade e das Áreas Protegidas (IBAP) Guinea Bissau; Ministry of Agriculture, Forestry and Food Security, Sierra Leone; Ministry of Education, Rwanda; National Protected Area Authority, Sierra Leone; Rwanda Development Board, Rwanda; Tanzania Commission for Science and Technology, Tanzania; Tanzania Wildlife Research Institute, Tanzania; Uganda National Council for Science and Technology (UNCST), Uganda; Uganda Wildlife Authority, Uganda; Makerere University Biological Field Station (MUBFS), Uganda; National Forestry Authority, Uganda. We thank Theophile Desarmeaux, Vianet Mihindou, Marcel Ketchan Eyong, Laura Kehoe, Lucy D’Auvergne, Nuria Maldonado, Jean Claude Dengui, Paul Telfer, Alhaji Malikie Siaka, Henk Eshuis, Geoffrey Muhanguzi, Yasmin Moebius, Nicolas Ntare, Vincent Lapeyre, Abel Nzeheke, Amelia Meier, Dervla Dowd, Luz Miramontes Sequeiros, Manuel Llana, Floris Aubert, Chloe Cipoletta, Katherine Corogenes, Emmanuel Dilambaka, Mohamed Kambi, Vera Leinert, Mizuki Murai, Emmanuelle Normand, Jodie Preece, Sebastien Regnaut, Emilien Terrade, Alexander Tickle, and Hilde Vanleeuwe, and Jane Goodall Institute volunteers for logistical support and/or sample collection. We also appreciate Claudia Herf, Christina Kompo, Claudia Feige, Andreas Walther, Rainer Benz for their administrative and IT support and Linda Vigilant, Anette Nicklish, Alan Riedel, and Katharina Madl for providing genetics laboratory support and assistance. We thank Fabian Leendertz for helpful feedback on this paper.
Contributor Information
Clifton P. Bueno de Mesquita, Email: cliff.buenodemesquita@colorado.edu.
Sarah M. Hird, University of Connecticut
REFERENCES
- 1.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryan JF, Dinan TG. 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 3.Blaser MJ, Dominguez-Bello MG. 2016. The human microbiome before birth. Cell Host Microbe 20:558–560. doi: 10.1016/j.chom.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. 2015. The infant microbiome development: mom matters. Trends Mol Med 21:109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung J, Barreiro LB, Burns MB, Grenier JC, Lynch J, Grieneisen LE, Altmann J, Alberts SC, Blekhman R, Archie EA. 2015. Social networks predict gut microbiome composition in wild baboons. Elife 4:e05224. doi: 10.7554/eLife.05224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar A, Harty S, Johnson KVA, Moeller AH, Archie EA, Schell LD, Carmody RN, Clutton-Brock TH, Dunbar RIM, Burnet PWJ. 2020. Microbial transmission in animal social networks and the social microbiome. Nat Ecol Evol 4:1020−1035. doi: 10.1038/s41559-020-1220-8. [DOI] [PubMed] [Google Scholar]
- 7.Grieneisen LE, Charpentier MJE, Alberts SC, Blekhman R, Bradburd G, Tung J, Archie EA. 2019. Genes, geology and germs: gut microbiota across a primate hybrid zone are explained by site soil properties, not host species. Proc R Soc B Biol Sci 286:20190431. doi: 10.1098/rspb.2019.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palopoli MF, Fergus DJ, Minot S, Pei DT, Simison WB, Fernandez-Silva I, Thoemmes MS, Dunn RR, Trautwein M. 2015. Global divergence of the human follicle mite Demodex folliculorum: persistent associations between host ancestry and mite lineages. Proc Natl Acad Sci U S A 112:15958–15963. doi: 10.1073/pnas.1512609112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson KP, Allen JM, Olds BP, Mugisha L, Reed DL, Paige KN, Pittendrigh BR. 2014. Rates of genomic divergence in humans, chimpanzees and their lice. Proc R Soc B 281:20132174. doi: 10.1098/rspb.2013.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris NC, Coonan TJ, King JL, Dunn RR. 2013. Endemism in host-parasite interactions among island populations of an endangered species. Divers Distrib 19:377–385. doi: 10.1111/ddi.12016. [DOI] [Google Scholar]
- 11.Greve M, Lykke AM, Blach-Overgaard A, Svenning JC. 2011. Environmental and anthropogenic determinants of vegetation distribution across Africa. Glob Ecol Biogeogr 20:661–674. doi: 10.1111/j.1466-8238.2011.00666.x. [DOI] [Google Scholar]
- 12.Wessling EG, Oelze VM, Eshuis H, Pruetz JD, Kühl HS. 2019. Stable isotope variation in savanna chimpanzees (Pan troglodytes verus) indicate avoidance of energetic challenges through dietary compensation at the limits of the range. Am J Phys Anthropol 168:665–675. doi: 10.1002/ajpa.23782. [DOI] [PubMed] [Google Scholar]
- 13.Nunn CL, Altizer SM, Sechrest W, Cunningham AA. 2005. Latitudinal gradients of parasite species richness in primates. Divers Distrib 11:249–256. doi: 10.1111/j.1366-9516.2005.00160.x. [DOI] [Google Scholar]
- 14.Hawley DM, Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol 25:48–60. doi: 10.1111/j.1365-2435.2010.01753.x. [DOI] [Google Scholar]
- 15.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gogarten JF, Davies TJ, Benjamino J, Gogarten JP, Graf J, Mielke A, Mundry R, Nelson MC, Wittig RM, Leendertz FH, Calvignac-Spencer S. 2018. Factors influencing bacterial microbiome composition in a wild non-human primate community in Taï National Park, Côte d’Ivoire. ISME J 12:2559–2574. doi: 10.1038/s41396-018-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, DeRight Goldasich L, Dorrestein PC, Dunn RR, Fahimipour AK, Gaffney J, Gilbert JA, Gogul G, Green JL, Hugenholtz P, Humphrey G, Huttenhower C, Jackson MA, Janssen S, Jeste DV, Jiang L, Kelley ST, Knights D, Kosciolek T, Ladau J, Leach J, Marotz C, Meleshko D, Melnik AV, Metcalf JL, Mohimani H, Montassier E, Navas-Molina J, Nguyen TT, Peddada S, Pevzner P, Pollard KS, Rahnavard G, Robbins-Pianka A, Sangwan N, Shorenstein J, Smarr L, Song SJ, Spector T, Swafford AD, Thackray VG, Thompson LR, et al. 2018. American Gut: an open platform for citizen science microbiome research. mSystems 3:e00031-18. doi: 10.1128/mSystems.00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maukonen J, Saarela M. 2015. Human gut microbiota: does diet matter? Proc Nutr Soc 74:23–36. doi: 10.1017/S0029665114000688. [DOI] [PubMed] [Google Scholar]
- 19.Amato KR, Mallott EK, McDonald D, Dominy NJ, Goldberg T, Lambert JE, Swedell L, Metcalf JL, Gomez A, Britton GAO, Stumpf RM, Leigh SR, Knight R. 2019. Convergence of human and Old World monkey gut microbiomes demonstrates the importance of human ecology over phylogeny. Genome Biol 20:201. doi: 10.1186/s13059-019-1807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prado-Martinez J, Sudmant PH, Kidd JM, Li H, Kelley JL, Lorente-Galdos B, Veeramah KR, Woerner AE, O’Connor TD, Santpere G, Cagan A, Theunert C, Casals F, Laayouni H, Munch K, Hobolth A, Halager AE, Malig M, Hernandez-Rodriguez J, Hernando-Herraez I, Prüfer K, Pybus M, Johnstone L, Lachmann M, Alkan C, Twigg D, Petit N, Baker C, Hormozdiari F, Fernandez-Callejo M, Dabad M, Wilson ML, Stevison L, Camprub C, Carvalho T, Ruiz-Herrera A, Vives L, Mele M, Abello T, Kondova I, Bontrop RE, Pusey A, Lankester F, Kiyang JA, Bergl RA, Lonsdorf E, Myers S, Ventura M, Gagneux P, Comas D, et al. 2013. Great ape genetic diversity and population history. Nature 499:471–475. doi: 10.1038/nature12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Leeuwen KL, Hill RA, Korstjens AH. 2020. Classifying chimpanzee (Pan troglodytes) landscapes across large-scale environmental gradients in Africa. Int J Primatol 41:800–821. doi: 10.1007/s10764-020-00164-5. [DOI] [Google Scholar]
- 22.Bogart SL, Pruetz JD. 2011. Insectivory of savanna chimpanzees (Pan troglodytes verus) at Fongoli, Senegal. Am J Phys Anthropol 145:11–20. doi: 10.1002/ajpa.21452. [DOI] [PubMed] [Google Scholar]
- 23.Oelze VM, Fahy G, Hohmann G, Robbins MM, Leinert V, Lee K, Eshuis H, Seiler N, Wessling EG, Head J, Boesch C, Kühl HS. 2016. Comparative isotope ecology of African great apes. J Hum Evol 101:1–16. doi: 10.1016/j.jhevol.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Piel AK, Strampelli P, Greathead E, Hernandez-Aguilar RA, Moore J, Stewart FA. 2017. The diet of open-habitat chimpanzees (Pan troglodytes schweinfurthii) in the Issa valley, western Tanzania. J Hum Evol 112:57–69. doi: 10.1016/j.jhevol.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Moore J, Black J, Hernandez-Aguilar RA, Idani G, Piel A, Stewart F. 2017. Chimpanzee vertebrate consumption: savanna and forest chimpanzees compared. J Hum Evol 112:30–40. doi: 10.1016/j.jhevol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Watts DP. 2020. Meat eating by nonhuman primates: a review and synthesis. J Hum Evol 149:102882. doi: 10.1016/j.jhevol.2020.102882. [DOI] [PubMed] [Google Scholar]
- 27.Loudon JE, Sandberg PA, Wrangham RW, Fahey B, Sponheimer M. 2016. The stable isotope ecology of Pan in Uganda and beyond. Am J Primatol 78:1070–1085. doi: 10.1002/ajp.22552. [DOI] [PubMed] [Google Scholar]
- 28.Nishida T, Hiraiwa M. 1982. Natural history of a tool-using behavior by wild chimpanzees in feeding upon wood-boring ants. J Hum Evol 11:73–99. doi: 10.1016/S0047-2484(82)80033-X. [DOI] [Google Scholar]
- 29.Boesch C, Marchesi P, Marchesi N, Fruth B, Joulian F. 1994. Is nut cracking in wild chimpanzees a cultural behaviour? J Hum Evol 26:325–338. doi: 10.1006/jhev.1994.1020. [DOI] [Google Scholar]
- 30.Boesch C, Kalan AK, Agbor A, Arandjelovic M, Dieguez P, Lapeyre V, Kühl HS. 2017. Chimpanzees routinely fish for algae with tools during the dry season in Bakoun, Guinea. Am J Primatol 79:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Luncz LV, Boesch C. 2015. The extent of cultural variation between adjacent chimpanzee (Pan troglodytes verus) communities; a microecological approach. Am J Phys Anthropol 156:67–75. doi: 10.1002/ajpa.22628. [DOI] [PubMed] [Google Scholar]
- 32.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Belknap Press, Cambridge, MA. [Google Scholar]
- 33.Sanz CM, Deblauwe I, Tagg N, Morgan DB. 2014. Insect prey characteristics affecting regional variation in chimpanzee tool use. J Hum Evol 71:28–37. doi: 10.1016/j.jhevol.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 34.McGrew WC. 1992. Chimpanzee material culture: implications for human evolution. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 35.Watts DP, Potts KP, Lwanga JS, Mitani JC. 2012. Diet of chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. 1. Diet composition and diversity. Am J Primatol 74:114–129. doi: 10.1002/ajp.21016. [DOI] [PubMed] [Google Scholar]
- 36.Sanz CM, Morgan DB. 2009. Flexible and persistent tool-using strategies in honey-gathering by wild chimpanzees. Int J Primatol 30:411–427. doi: 10.1007/s10764-009-9350-5. [DOI] [Google Scholar]
- 37.Matsuzawa T. 2019. Chimpanzees foraging on aquatic foods: algae scooping in Bossou. Primates 60:317–319. doi: 10.1007/s10329-019-00733-0. [DOI] [PubMed] [Google Scholar]
- 38.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. 1999. Cultures in chimpanzees. Nature 399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 39.Kühl HS, Boesch C, Kulik L, Haas F, Arandjelovic M, Dieguez P, Bocksberger G, McElreath MB, Agbor A, Angedakin S, Ayimisin EA, Bailey E, Barubiyo D, Bessone M, Brazzola G, Chancellor R, Cohen H, Coupland C, Danquah E, Deschner T, Dowd D, Dunn A, Egbe VE, Eshuis H, Goedmakers A, Granjon AC, Head J, Hedwig D, Hermans V, Imong I, Jeffery KJ, Jones S, Junker J, Kadam P, Kambere M, Kambi M, Kienast I, Kujirakwinja D, Langergraber KE, Lapuente J, Larson B, Lee K, Leinert V, Llana M, Maretti G, Marrocoli S, Martin R, Mbi TJ, Meier AC, Morgan B, et al. 2019. Human impact erodes chimpanzee behavioral diversity. Science 363:1453–1455. doi: 10.1126/science.aau4532. [DOI] [PubMed] [Google Scholar]
- 40.Lester JD, Vigilant L, Gratton P, McCarthy MS, Barratt CD, Dieguez P, Agbor A, Álvarez-Varona P, Angedakin S, Ayimisin EA, Bailey E, Bessone M, Brazzola G, Chancellor R, Cohen H, Danquah E, Deschner T, Egbe VE, Eno-Nku M, Goedmakers A, Granjon A-C, Head J, Hedwig D, Hernandez-Aguilar A, Jeffery KJ, Jones S, Junker J, Kadam P, Kaiser M, Kalan AK, Kehoe L, Kienast I, Langergraber KE, Lapuente J, Laudisoit A, Lee K, Marrocoli S, Mihindou V, Morgan D, Muhanguzi G, Neil E, Nicholl S, Orbell C, Ormsby LJ, Pacheco L, Piel A, Robbins M, Rundus A, Sanz C, Sciaky L, et al. 2021. Recent genetic connectivity and clinal variation in chimpanzees. Commun Biol 4:283. doi: 10.1038/s42003-021-01806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amato KR. 2019. Missing links: the role of primates in understanding the human microbiome. mSystems 4:e00165-19. doi: 10.1128/mSystems.00165-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishida AH, Ochman H. 2019. A great-ape view of the gut microbiome. Nat Rev Genet 20:195–206. doi: 10.1038/s41576-018-0085-z. [DOI] [PubMed] [Google Scholar]
- 43.Hansen MEB, Rubel MA, Bailey AG, Ranciaro A, Thompson SR, Campbell MC, Beggs W, Dave JR, Mokone GG, Mpoloka SW, Nyambo T, Abnet C, Chanock SJ, Bushman FD, Tishkoff SA. 2019. Population structure of human gut bacteria in a diverse cohort from rural Tanzania and Botswana. Genome Biol 20:16. doi: 10.1186/s13059-018-1616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hicks AL, Lee KJ, Couto-Rodriguez M, Patel J, Sinha R, Guo C, Olson SH, Seimon A, Seimon TA, Ondzie AU, Karesh WB, Reed P, Cameron KN, Lipkin WI, Williams BL. 2018. Gut microbiomes of wild great apes fluctuate seasonally in response to diet. Nat Commun 9:1786. doi: 10.1038/s41467-018-04204-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-González A, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK, Fierer N. 2018. A global atlas of the dominant bacteria found in soil. Science 359:320–325. doi: 10.1126/science.aap9516. [DOI] [PubMed] [Google Scholar]
- 46.Vorholt JA. 2012. Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 47.Gonder MK, Locatelli S, Ghobrial L, Mitchell MW, Kujawski JT, Lankester FJ, Stewart CB, Tishkoff SA. 2011. Evidence from Cameroon reveals differences in the genetic structure and histories of chimpanzee populations. Proc Natl Acad Sci U S A 108:4766–4771. doi: 10.1073/pnas.1015422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Cáceres M, Legendre P. 2009. Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. doi: 10.1890/08-1823.1. [DOI] [PubMed] [Google Scholar]
- 49.Sommer V, Buba U, Gonçalo J, Pascual-Garrido A. 2012. Till the last drop: honey gathering in Nigerian chimpanzees. Ecotropica 18:55–64. [Google Scholar]
- 50.Sommer V, Ross C (ed). 2011. Primates of Gashaka: sociology and conservation in Nigeria’s biodiversity hotspot. Springer, New York, NY. [Google Scholar]
- 51.Reynolds V. 2005. The chimpanzees of Budongo Forest: ecology, behaviour and conservation. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 52.Newton-Fisher N. 1999. Termite eating and food sharing by male chimpanzees in the Budongo Forest, Uganda. Afr J Ecol 37:369–371. doi: 10.1046/j.1365-2028.1999.00187.x. [DOI] [Google Scholar]
- 53.Sanz CM, Morgan DB. 2007. Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J Hum Evol 52:420–433. doi: 10.1016/j.jhevol.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Estienne V, Stephens C, Boesch C. 2017. Extraction of honey from underground bee nests by central African chimpanzees (Pan troglodytes troglodytes) in Loango National Park, Gabon: techniques and individual differences. Am J Primatol 79:e22672. doi: 10.1002/ajp.22672. [DOI] [PubMed] [Google Scholar]
- 55.Martínez I, Stegen JC, Maldonado-Gómez MX, Eren MA, Siba PM, Greenhill AR, Walter J. 2015. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep 11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 56.Nogués-Bravo D, Araújo MB, Romdal T, Rahbek C. 2008. Scale effects and human impact on the elevational species richness gradients. Nature 453:216–219. doi: 10.1038/nature06812. [DOI] [PubMed] [Google Scholar]
- 57.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, Lawley TD, Finn RD. 2019. A new genomic blueprint of the human gut microbiota. Nature 568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, Beghini F, Manghi P, Tett A, Ghensi P, Collado MC, Rice BL, DuLong C, Morgan XC, Golden CD, Quince C, Huttenhower C, Segata N. 2019. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 176:649–662.e20. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nayfach S, Shi ZJ, Seshadri R, Pollard KS, Kyrpides NC. 2019. New insights from uncultivated genomes of the global human gut microbiome. Nature 568:505–510. doi: 10.1038/s41586-019-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature 555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 62.Moeller AH, Caro-Quintero A, Mjungu D, Georgiev AV, Lonsdorf EV, Muller MN, Pusey AE, Peeters M, Hahn BH, Ochman H. 2016. Cospeciation of gut microbiota with hominids. Science 353:380–382. doi: 10.1126/science.aaf3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moeller AH, Li Y, Ngole EM, Ahuka-Mundeke S, Lonsdorf EV, Pusey AE, Peeters M, Hahn BH, Ochman H. 2014. Rapid changes in the gut microbiome during human evolution. Proc Natl Acad Sci U S A 111:16431–16435. doi: 10.1073/pnas.1419136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moeller AH, Shilts M, Li Y, Rudicell RS, Lonsdorf EV, Pusey AE, Wilson ML, Hahn BH, Ochman H. 2013. SIV-induced instability of the chimpanzee gut microbiome. Cell Host Microbe 14:340–345. doi: 10.1016/j.chom.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nunn C, Altizer S. 2006. Infectious diseases in primates: behavior, ecology and evolution. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 66.Degnan PH, Pusey AE, Lonsdorf EV, Goodall J, Wroblewski EE, Wilson ML, Rudicell RS, Hahn BH, Ochman H. 2012. Factors associated with the diversification of the gut microbial communities within chimpanzees from Gombe National Park. Proc Natl Acad Sci U S A 109:13034–13039. doi: 10.1073/pnas.1110994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moeller AH, Foerster S, Wilson ML, Pusey AE, Hahn BH, Ochman H. 2016. Social behavior shapes the chimpanzee pan-microbiome. Sci Adv 2:e1500997. doi: 10.1126/sciadv.1500997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gogarten JF, Rühlemann M, Archie E, Tung J, Akoua-Koffi C, Bang C, Deschner T, Muyembe-Tamfun J-J, Robbins MM, Schubert G, Surbeck M, Wittig RM, Zuberbühler K, Baines JF, Franke A, Leendertz FH, Calvignac-Spencer S. 2021. Primate phageomes are structured by superhost phylogeny and environment. Proc Natl Acad Sci U S A 118:e2013535118. doi: 10.1073/pnas.2013535118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amato KR, Van Belle S, Fiore AD, Estrada A, Stumpf R, White B, Nelson KE, Knight R, Leigh SR. 2017. Patterns in gut microbiota similarity associated with degree of sociality among sex classes of a neotropical primate. Microb Ecol 74:250–258. doi: 10.1007/s00248-017-0938-6. [DOI] [PubMed] [Google Scholar]
- 70.Perofsky AC, Lewis RJ, Abondano LA, Fiore AD, Meyers LA. 2017. Hierarchical social networks shape gut microbial composition in wild Verreaux’s sifaka. Proc R Soc B 284:20172274. doi: 10.1098/rspb.2017.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barelli C, Albanese D, Stumpf RM, Asangba A, Donati C, Rovero F, Hauffe HC. 2020. The gut microbiota communities of wild arboreal and ground-feeding tropical primates are affected differently by habitat disturbance. mSystems 5:e00061-20. doi: 10.1128/mSystems.00061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï Forest: behavioural ecology and evolution. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 73.Möbius Y, Boesch C, Koops K, Matsuzawa T, Humle T. 2008. Cultural differences in army ant predation by West African chimpanzees? A comparative study of microecological variables. Anim Behav 76:37–45. doi: 10.1016/j.anbehav.2008.01.008. [DOI] [Google Scholar]
- 74.Amato KR, Martinez-Mota R, Righini N, Raguet-Schofield M, Corcione FP, Marini E, Humphrey G, Gogul G, Gaffney J, Lovelace E, Williams LS, Luong A, Dominguez-Bello MG, Stumpf RM, White B, Nelson KE, Knight R, Leigh SR. 2016. Phylogenetic and ecological factors impact the gut microbiota of two Neotropical primate species. Oecologia 180:717–733. doi: 10.1007/s00442-015-3507-z. [DOI] [PubMed] [Google Scholar]
- 75.Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. 2010. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- 76.McLennan MR. 2015. Is honey a fallback food for wild chimpanzees or just a sweet treat? Am J Phys Anthropol 158:685–695. doi: 10.1002/ajpa.22824. [DOI] [PubMed] [Google Scholar]
- 77.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W. 2017. Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamakoshi G. 1998. Dietary responses to fruit scarcity of wild chimpanzees at Bossou, Guinea: possible implications for ecological importance of tool use. Am J Phys Anthropol 106:283–295. doi:. [DOI] [PubMed] [Google Scholar]
- 79.O’Malley RC, Power ML. 2014. The energetic and nutritional yields from insectivory for Kasekela chimpanzees. J Hum Evol 71:46–58. doi: 10.1016/j.jhevol.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Pika S, Klein H, Bunel S, Baas P, Théleste E, Deschner T. 2019. Wild chimpanzees (Pan troglodytes troglodytes) exploit tortoises (Kinixys erosa) via percussive technology. Sci Rep 9:7661. doi: 10.1038/s41598-019-43301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–109. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacIntosh AJJ, Jacobs A, Garcia C, Shimizu K, Mouri K, Huffman MA, Hernandez AD. 2012. Monkeys in the middle: parasite transmission through the social network of a wild primate. PLoS One 7:e51144. doi: 10.1371/journal.pone.0051144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Renelies-Hamilton J, Noguera-Julian M, Parera M, Paredes R, Pacheco L, Dacal E, Saugar JM, Rubio JM, Poulsen M, Köster PC, Carmena D. 2019. Exploring interactions between Blastocystis sp., Strongyloides spp. and the gut microbiomes of wild chimpanzees in Senegal. Infect Genet Evol 74:104010. doi: 10.1016/j.meegid.2019.104010. [DOI] [PubMed] [Google Scholar]
- 84.Petrželková KJ, Smejkalová P, Céza V, Pafčo B, Shutt-Phillips KA, Todd A, Jirků-Pomajbíková K, Benavides J, Modrý D, Čepička I. 2020. Sympatric western lowland gorillas, central chimpanzees, and humans are infected with different trichomonads. Parasitology 147:225–230. doi: 10.1017/S0031182019001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mann AE, Mazel F, Lemay MA, Morien E, Billy V, Kowalewski M, Di Fiore A, Link A, Goldberg TL, Tecot S, Baden AL, Gomez A, Sauther ML, Cuozzo FP, Rice GAO, Dominy NJ, Stumpf R, Lewis RJ, Swedell L, Amato K, Wegener Parfrey L. 2020. Biodiversity of protists and nematodes in the wild nonhuman primate gut. ISME J 14:609–622. doi: 10.1038/s41396-019-0551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gogarten JF, Calvignac-Spencer S, Nunn CL, Ulrich M, Saiepour N, Nielsen HV, Deschner T, Fichtel C, Kappeler PM, Knauf S, Müller-Klein N, Ostner J, Robbins MM, Sangmaneedet S, Schülke O, Surbeck M, Wittig RM, Sliwa A, Strube C, Leendertz FH, Roos C, Noll A. 2020. Metabarcoding of eukaryotic parasite communities describes diverse parasite assemblages spanning the primate phylogeny. Mol Ecol Resour 20:204–215. doi: 10.1111/1755-0998.13101. [DOI] [PubMed] [Google Scholar]
- 87.Rushmore J, Bisanzio D, Gillespie TR. 2017. Making new connections: insights from primate–parasite networks. Trends Parasitol 33:547–560. doi: 10.1016/j.pt.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 88.Nunn CL, Altizer SM. 2005. The global mammal parasite database: an online resource for infectious disease records in wild primates. Evol Anthropol 14:1–2. doi: 10.1002/evan.20041. [DOI] [Google Scholar]
- 89.Profousová I, Mihaliková K, Laho T, Váradyová Z, Petrželková KJ, Modrý D, Kišidayová S. 2011. The ciliate, Troglodytella abrassarti, contributes to polysaccharide hydrolytic activities in the chimpanzee colon. Folia Microbiol (Praha) 56:339–343. doi: 10.1007/s12223-011-0053-x. [DOI] [PubMed] [Google Scholar]
- 90.Rimbach R, Bisanzio D, Galvis N, Link A, Fiore AD, Gillespie TR. 2015. Brown spider monkeys (Ateles hybridus): a model for differentiating the role of social networks and physical contact on parasite transmission dynamics. Philos Trans R Soc B Biol Sci 370:20140110. doi: 10.1098/rstb.2014.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Friant S, Ziegler TE, Goldberg TL. 2016. Primate reinfection with gastrointestinal parasites: behavioural and physiological predictors of parasite acquisition. Anim Behav 117:105–113. doi: 10.1016/j.anbehav.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Čepička I, Hampl V, Kulda J, Flegr J. 2006. New evolutionary lineages, unexpected diversity, and host specificity in the parabasalid genus Tetratrichomonas. Mol Phylogenet Evol 39:542–551. doi: 10.1016/j.ympev.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 93.De Nys HM, Madinda NF, Merkel K, Robbins M, Boesch C, Leendertz FH, Calvignac-Spencer S. 2015. A cautionary note on fecal sampling and molecular epidemiology in predatory wild great apes. Am J Primatol 77:833–840. doi: 10.1002/ajp.22418. [DOI] [PubMed] [Google Scholar]
- 94.Navarro-Gonzalez N, Verheyden H, Hoste H, Cargnelutti B, Lourtet B, Merlet J, Daufresne T, Lavín S, Hewison AJM, Morand S, Serrano E. 2011. Diet quality and immunocompetence influence parasite load of roe deer in a fragmented landscape. Eur J Wildl Res 57:639–645. doi: 10.1007/s10344-010-0474-x. [DOI] [Google Scholar]
- 95.Ezenwa VO. 2004. Interactions among host diet, nutritional status and gastrointestinal parasite infection in wild bovids. Int J Parasitol 34:535–542. doi: 10.1016/j.ijpara.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 96.Ratcliffe H. 1928. The numbers of trichomonads in rats on diets of different protein content in relation to the pH and bacteria in the cecum. Am J Hyg 8:910–934. doi: 10.1093/oxfordjournals.aje.a121038. [DOI] [Google Scholar]
- 97.Morton ER, Lynch J, Froment A, Lafosse S, Heyer E, Przeworski M, Blekhman R, Ségurel L. 2015. Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and subsistence. PLoS Genet 11:e1005658. doi: 10.1371/journal.pgen.1005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stensvold CR, van der Giezen M. 2018. Associations between gut microbiota and common luminal intestinal parasites. Trends Parasitol 34:369–377. doi: 10.1016/j.pt.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 99.Leung JM, Graham AL, Knowles SCL. 2018. Parasite-microbiota interactions with the vertebrate gut: synthesis through an ecological lens. Front Microbiol 9:843. doi: 10.3389/fmicb.2018.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newbold CJ, de la Fuente G, Belanche A, Ramos-Morales E, McEwan NR. 2015. The role of ciliate protozoa in the rumen. Front Microbiol 6:1313. doi: 10.3389/fmicb.2015.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lukeš J, Stensvold CR, Jirků-Pomajbíková K, Wegener Parfrey L. 2015. Are human intestinal eukaryotes beneficial or commensals? PLoS Pathog 11:e1005039. doi: 10.1371/journal.ppat.1005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Méthot PO, Alizon S. 2014. What is a pathogen? Toward a process view of host-parasite interactions. Virulence 5:775–785. doi: 10.4161/21505594.2014.960726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arandjelovic M, Boesch C, Campbell G, Hohmann G, Junker J, Kouakou YC, Kühl H, Leendertz F, Leinert V, Möbius Y, Murai M, Oelze V, Rabanal L, Robbina M, Vergnes V, Wagner O, Head J. 2020. Pan African Programme: the cultured chimpanzee. Guidelines for Research and Data Collection.
- 104.Aleman JC, Fayolle A, Favier C, Staver AC, Dexter KG, Ryan CM, Azihou AF, Bauman D, Te Beest M, Chidumayo EN, Comiskey JA, Cromsigt JPGM, Dessard H, Doucet JL, Finckh M, Gillet JF, Gourlet-Fleury S, Hempson GP, Holdo RM, Kirunda B, Kouame FN, Mahy G, Maiato F, Gonçalves P, McNicol I, Nieto Quintano P, Plumptre AJ, Pritchard RC, Revermann R, Schmitt CB, Swemmer AM, Talila H, Woollen E, Swaine MD. 2020. Floristic evidence for alternative biome states in tropical Africa. Proc Natl Acad Sci U S A 117:28183–28190. doi: 10.1073/pnas.2011515117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalan AK, Kulik L, Arandjelovic M, Boesch C, Haas F, Dieguez P, Barratt CD, Abwe EE, Agbor A, Angedakin S, Aubert F, Ayimisin EA, Bailey E, Bessone M, Brazzola G, Buh VE, Chancellor R, Cohen H, Coupland C, Curran B, Danquah E, Deschner T, Dowd D, Eno-Nku M, Michael Fay J, Goedmakers A, Granjon AC, Head J, Hedwig D, Hermans V, Jeffery KJ, Jones S, Junker J, Kadam P, Kambi M, Kienast I, Kujirakwinja D, Langergraber KE, Lapuente J, Larson B, Lee KC, Leinert V, Llana M, Marrocoli S, Meier AC, Morgan B, Morgan D, Neil E, Nicholl S, Normand E, et al. 2020. Environmental variability supports chimpanzee behavioural diversity. Nat Commun 11:4451. doi: 10.1038/s41467-020-18176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nsubuga AM, Robbins MM, Roeder AD, Morin PA, Boesch C, Vigilant L. 2004. Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Mol Ecol 13:2089–2094. doi: 10.1111/j.1365-294X.2004.02207.x. [DOI] [PubMed] [Google Scholar]
- 107.Phillips CA, Woolley C, Mann D, McGrew WC. 2018. Disappearance rate of chimpanzee scats: implications for census work on Pan troglodytes. Afr J Ecol 56:168–178. doi: 10.1111/aje.12501. [DOI] [Google Scholar]
- 108.Vigilant L. 2003. Technical challenges in the microsatellite genotyping of a wild chimpanzee population using feces. Evol Anthropol 11:162–165. doi: 10.1002/evan.10082. [DOI] [Google Scholar]
- 109.Arandjelovic M, Guschanski K, Schubert G, Harris TR, Thalmann O, Siedel H, Vigilant L. 2009. Two-step multiplex polymerase chain reaction improves the speed and accuracy of genotyping using DNA from noninvasive and museum samples. Mol Ecol Resour 9:28–36. doi: 10.1111/j.1755-0998.2008.02387.x. [DOI] [PubMed] [Google Scholar]
- 110.Waits LP, Luikart G, Taberlet P. 2001. Estimating the probability of identity among genotypes in natural populations: cautions and guidelines. Mol Ecol 10:249–256. doi: 10.1046/j.1365-294x.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- 111.Smouse PE, Peakall R. 1999. Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity (Edinb) 82:561–573. doi: 10.1038/sj.hdy.6885180. [DOI] [PubMed] [Google Scholar]
- 112.Meirmans PG. 2020. genodive version 3.0: easy‐to‐use software for the analysis of genetic data of diploids and polyploids. Mol Ecol Resour 20:1126–1131. doi: 10.1111/1755-0998.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R, Earth Microbiome Project Consortium . 2017. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal 17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 115.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callahan BJ, McMurdie PJ, Holmes SP. 2017. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J 11:2639–2643. doi: 10.1038/ismej.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guillou L, Bachar D, Audic S, Bass D, Berney C, Bittner L, Boutte C, Burgaud G, de Vargas C, Decelle J, del Campo J, Dolan J, Dunthorn M, Edvardsen B, Holzmann M, Kooistra W, Lara E, Bescot NL, Logares R, Mahé F, Massana R, Montresor M, Morard R, Not F, Pawlowski J, Probert I, Sauvadet A, Siano R, Stoeck T, Vaulot D, Zimmermann P, Christen R. 2013. The Protist Ribosomal Reference database (PR2): a catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res 41:D597–D604. doi: 10.1093/nar/gks1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caporaso JG, Bittinger K, Bushman FD, Desantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pẽa AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335−336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu G, Smith D, Zhu H, Guan Y, Lam TT-Y. 2017. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 123.Leff JW. 2017. mctoolsr: microbial community data analysis tools. R package version 0.1.1.2.
- 124.Ashford RW, Reid GDF, Wrangham RW. 2000. Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Ann Trop Med Parasitol 94:173–179. doi: 10.1080/00034983.2000.11813526. [DOI] [PubMed] [Google Scholar]
- 125.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. doi: 10.1046/j.1442-9993.2001.01070.x. [DOI] [Google Scholar]
- 126.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH. 2019. vegan: community ecology package. R package version 2.5-6.
- 127.Hervé M. 2019. RVAideMemoire: testing and plotting procedures for biostatistics. R package version 0.9-73. https://cran.r-project.org/package=RVAideMemoire.
- 128.Ferrier S, Manion G, Elith J, Richardson K. 2007. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers Distrib 13:252–264. doi: 10.1111/j.1472-4642.2007.00341.x. [DOI] [Google Scholar]
- 129.Fitzpatrick M, Mokany K, Glenn M, Lisk M, Simon F, Nieto-Lugilde D. 2020. gdm: generalized dissimilarity modeling. R package version 1.4.2.
- 130.Moeller AH, Degnan PH, Pusey AE, Wilson ML, Hahn BH, Ochman H. 2012. Chimpanzees and humans harbour compositionally similar gut enterotypes. Nat Commun 3:1179. doi: 10.1038/ncomms2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Campbell TP, Sun X, Patel VH, Sanz C, Morgan D, Dantas G. 2020. The microbiome and resistome of chimpanzees, gorillas, and humans across host lifestyle and geography. ISME J 14:1584–1599. doi: 10.1038/s41396-020-0634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amir A, McDonald D, Navas-Molina JA, Kopylova E, Morton JT, Zech Xu Z, Kightley EP, Thompson LR, Hyde ER, Gonzalez A, Knight R. 2017. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2:e00191-16. doi: 10.1128/mSystems.00191-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Humle T, Maisels F, Oates JF, Plumptre A, Williamson EA. 2016. Pan troglodytes (errata version published in 2018). The IUCN Red List of Threatened Species 2016: e.T15933A129038584. 10.2305/IUCN.UK.2016-2.RLTS.T15933A17964454.en. Downloaded 31 Mar 2020. [DOI]
- 134.Myers BJ, Kuntz RE. 1972. A checklist of parasites and commensals reported for the chimpanzee (Pan). Primates 13:433–471. doi: 10.1007/BF01793663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data and ASV sequences were deposited to NCBI SRA and GenBank, respectively, under the BioProject accession number PRJNA625726.