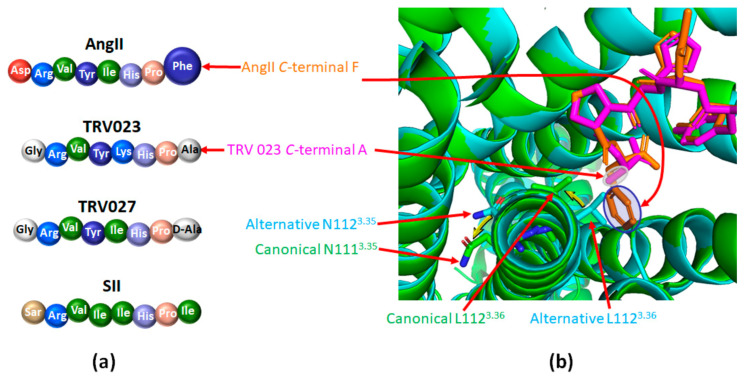
Figure 6.
β-arrestin biased agonists of AT1 favor its alternative active conformation. (a): Comparison of the amino acid sequences of angiotensin II (Ang II) and of the main β-arrestin biased agonists of AT1; Ang II: angiotensin II; SII: [Sar1,Ile4,Ile8] angiotensin II peptide analog; TRV023 and TRV027: β-arrestin biased AT1 agonists. (b): Superimposition of the structure of AT1 bound to Ang II (green, PDB code 6OS0; Ang II appears in orange) or to the β-arrestin biased agonist TRV023 (cyan, PDB code 6OS1; TRV023 appears in purple). The phenyl group of the C-terminal part of Ang II (highlighted in blue) goes deep into the ligand pocket, inducing a rotation of L1123.36 and of N1113.35 (yellow arrows). It yields the canonical structure of AT1 (appears in green). The C-terminal part of TRV023 comprises a less bulky alanine residue (highlighted in grey) which does not induce such motion, yielding the alternative structure (in cyan). This panel was prepared with the PyMOL software (The PyMOL Molecular Graphics System, Version 2.5.0. Schrödinger, LLC).