Purpose of review
Whereas the COVID-19 pandemic has changed our lives worldwide, we hope that vaccination can combat the disease. We propose how to evaluate suspected severe allergic reactions to the vaccines so that as many as possible may be safely vaccinated.
Recent findings
Rare cases of severe allergic reactions after COVID-19 vaccination have been observed, seemingly at a higher frequency than for other vaccines. Few excipients are likely to have caused these reactions. IgE-mediated reactions to polyethylene glycol (PEG) and its derivatives are the most suspected, albeit hitherto unproven, causes. We suggest to make a diagnosis based on skin tests with PEG and PEG derivatives and that these be considered in relation to the decisions required before the first and the second vaccine dose. A vaccine without these excipients is available, but published data about its side effects are limited.
Summary
The underlying immunological mechanisms of the rare severe allergic reactions to the COVID-19 vaccines are poorly understood and need to be clarified. Identifying those who have an undiagnosed allergy to PEG and PEG derivatives is crucial before vaccination, and these substances are found in laxatives, cosmetics and in 30% of all our medications today.
Keywords: allergic reactions, anaphylaxis, COVID-19 vaccines, polyethylene glycol
INTRODUCTION
During the ongoing mass vaccination against SARS-CoV-2, rare cases of immediate allergic reactions of anaphylaxis have been observed [1▪▪]. It is important that these patients are evaluated by an allergologist to obtain specific diagnoses and advice concerning future vaccinations. This article aims to facilitate evaluation of suspected serious allergic reactions to COVID-19 vaccines, including a thorough medical history and skin testing, with standard steps and guidelines.
The most severe allergic reaction to vaccines is anaphylaxis and different diagnostic criteria have been suggested. The widely used NIAID/FAAN criteria were published in 2006 [2] and has been updated by the World Allergy Organization (WAO) [3,4] for use in anaphylaxis of all causes. The Brighton Collaboration specifically developed guidelines, published in 2007, for adverse events after vaccinations. This guideline for anaphylaxis has three levels using a combination of symptoms to determine levels of diagnostic certainty rather than severity [5]. This is different from NIAID/FAAN and WAO's description, where the severity and the grading of the severity are determined. While the Brighton Collaboration system has now been widely adopted worldwide in the assessment of reactions to the COVID-19 vaccines, information on sensitivity and specificity of this approach is lacking.
Box 1.
no caption available
HISTORY OF VACCINATION AGAINST CORONAVIRUS INFECTION
Various coronaviruses cause symptomatic infections in domestic species such as dogs, cats, calves, pigs and chicken. Live and inactivated animal vaccines against coronaviruses have long been used in veterinary medicine, proving the feasibility of vaccination [6].
In humans, four different pathogenic human coronaviruses were identified in archived nasopharyngeal aspirates before 2002. The patients were typically reported to exhibit the mild respiratory symptoms known as the seasonal common cold. The viral strains are named HCoV-229E, HCoV-NL63, HCoV-OC43 and HCoV-HKU1 [7,8]. Successful human vaccines against these viruses have not been developed due to common mutations in these viral strains [9].
In 2002, a novel coronavirus, later named SARS-CoV, was reported from China in patients with atypical pneumonia and acute respiratory distress syndrome (ARDS). This virus had high mortality and spread worldwide, but the disease was contained with public health measures only. A number of vaccine companies started research and development programmes against SARS, but these were generally terminated when SARS was eradicated from the human population in 2004 [9]. Immunological studies in SARS survivors have shown lasting T-cell immunity after 17 years [10].
In 2012, another pathogenic novel coronavirus appeared in the Middle East region. This virus – known as MERS-CoV, it is linked to high mortality due to atypical pneumonia, ARDS and renal failure. It is still circulating locally, but human-to-human transmission is limited.
SARS-CoV-2 virus causes the COVID-19 infection and is the seventh coronavirus known to infect humans and Chinese investigators released the genomic sequences of SARS-CoV-2 isolates on 10 January 2020. As some research groups had already established MERS-CoV or SARS-CoV as prototype pathogens to optimize vaccine design, delivery mechanisms and correlates of protection work on a vaccine was initiated almost immediately. In this way, the first human clinical trials on mRNA-vaccine formulated in lipid nanoparticles started as early as 16 March 2020 [11]. In the following months, clinical trials commenced with replication-incompetent adenovirus vector vaccine candidates and inactivated virus vaccines, and in May 2020 a recombinant spike-protein-based vaccine candidate was evaluated in clinical trials [9] (Table 1).
Table 1.
COVID-19 vaccines mentioned in the text and used for mass vaccination in Europe
| Product type | Manufacturer and name | Suspected culprit allergen |
| mRNA vaccine | Pfizer/BioNTech, BNT16262, BioNTechManufacturing GmbH Mainz Germany and Pfizer Manufacturing GmbH Puurs, Belgium, ‘Comirnaty’ COVID-19 Vaccine Moderna, Moderna Biotech Spain, Madrid, Spain | Polyethylene glycol (PEG-2000) |
| Adenovirus vector vaccine | AstraZeneca, AZD1222, AstraZeneca AB, Södertälje, Sweden, ‘Vaxzevria’ COVID-19 Vaccine Janssen, Ad26.COV2-S, Janssen-Cilag International NV, Beerre, Belgium Sputnik V, Gamaleya Research Institute of Epidemiology and Mirobiology, Moscow, Russia | Polysorbate 80 |
| Inactivated virus | BBIBP-CorV, Beijing Institute of Biological Products, Beijing, China, ‘Sinopharm’ | |
| Recombinant spike protein | Novavax COVID-19 Vaccine, NVX-COV2373, Novavax Inc, Gaithersburg, Maryland, USA | Polysorbate 80 |
Note: BBIBP-CorV does not contain polyethylene glycol or polysorbate 80. PEG, polyethylene glycol.
SERIOUS ADVERSE AND ANAPHYLAXIS EVENTS IN MASS VACCINATION PROGRAMMES
After UK emergency approval of the mRNA vaccine produced by Pfizer/BioNTech, BNT16262, BioNTechManufacturing GmbH Mainz Germany and Pfizer Manufacturing GmbH Puurs, Belgium, “Comirnaty” (Table 1), mass vaccination of healthcare workers (HCWs) and high-risk patients against COVID-19 started on 8 December 2020. The next day two cases of anaphylaxis were reported among two female HCW. On 11 December, emergency use authorization was issued in the United States for Comirnaty, and six further cases were reported in the following week (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-Clark-508.pdf; [12▪▪,13]). Before the start of the mass vaccine programmes against COVID-19, serious allergic reactions and anaphylaxis in connection with vaccines were regarded as extremely rare adverse events, with an overall risk estimate of 1.31 per million doses and with rather narrow 95% confidence intervals of 0.9–1.8, defined by Brighton level 1 and 2. Reactions occurred most often in women with 80% being women [14,15]. Increased rates of severe reactions in women were also observed for the COVID-19 mRNA vaccines [12▪▪]. The severe hypersensitivity symptoms mostly started within 10–30 min of exposure. The most common symptoms were urticaria, itching, flushing, general discomfort, angioedema, breathlessness, burning sensation and fainting [12▪▪].
Studies have confirmed initial suspicions and suggested that the incidence of anaphylaxis after mRNA vaccine against COVID-19 is somewhat higher than previously expected, but with very low mortality. The initial Vaccine Adverse Event Reporting System (United States) estimate for Comirnaty using the international standard case definition from Brighton Collaboration [5] is 4.7 cases per 1 million vaccine doses [12▪▪]. Initial safety signals were based on spontaneous reports influenced by awareness of a potential safety concern for the vaccines, potential underestimation of the denominator but also increased focus on this first vaccine. Even for a more recently licensed mRNA vaccine against COVID-19 produced by Moderna, the incidence of anaphylaxis immediately postvaccination appears higher than expected, at 2.5 per million doses [16,17].
As of end of March 2021, the United Kingdom had vaccinated 10.9 million people with Pfizer's vaccine and had administered 13.7 million doses of AstraZeneca's (AstraZeneca, AZD1222, AstraZeneca AB, Södertälje, Sweden) chimpanzee adenovirus vaccine, containing double-stranded DNA coding for the spike protein of SARS-CoV-2 (Vaxzevria). For 9 December 2020 to 8 April 2021, no evident significant differences existed in the reporting rate for anaphylaxis (spontaneous reports); 259 reports for the Pfizer vaccine (24 per million doses) and 455 reports for the AstraZeneca's vaccine (23 per million doses) [18]. Information of side effects of the vaccines have been given by healthcare professionals.
The Russian adenovirus vector vaccine, Sputnik V, is based on a similar platform to the AstraZeneca vaccine (using two human adenoviral vectors, Ad5 and Ad26), and is currently being used for mass vaccination in Serbia and Hungary. Both countries have also used an inactivated whole virus vaccine, Sinopharm, BBIBP-CorV. The two latter vaccines have been used consistently for many months in mass vaccination programmes in Russia, Middle East and China, but hitherto no vaccine-specific data on serious adverse events and anaphylaxis post vaccination has been presented.
A recently licensed single-dose adenovirus vector (Ad26) vaccine from Janssen Vaccine will be used in European mass vaccination programs from April 2021. The vaccine is reported to be less reactogenic than the two mRNA vaccines used in the United States, and no reports of anaphylaxis have emerged from the clinical phase 2/phase 3 studies. However, no such reactions were reported in the phase 2/phase 3 trials for the mRNA vaccines from Pfizer/BioNTech and Moderna. Of note, in the phases 1–3 clinical trials of the Pfizer/BioNTech and Moderna mRNA vaccines, anyone with a history of allergic reaction to any component of the vaccine was excluded, as were those with a history of severe allergy associated with any vaccine [19,20]. Similar exclusion criteria were used in the Janssen and Astra Zeneca phase 3 trials (https://www.jnj.com/coronavirus/ensemble-1-study-protocol; https://www.thelancet.com/cms/10.1016/S0140-6736(20)32661-1/attachment/87a97e27-03d4-40c9-8498-7e513e08d265/mmc2.pdf).
EXCIPENTS IN THE VACCINES POTENTIALLY CAUSING ANAPHYLAXIS
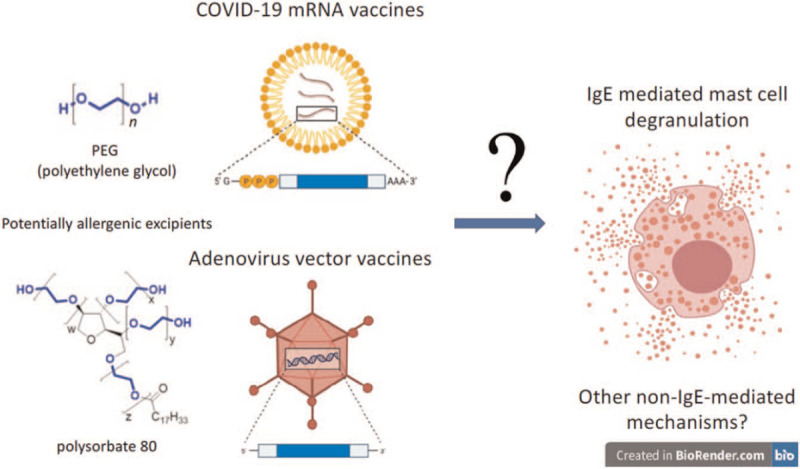
Allergic reactions to vaccines are usually caused by an inactive ingredient or excipient [21]. It is still unclear whether excipients caused the anaphylactic reactions observed after administration of the COVID-19 vaccines [1▪▪]. However, polyethylene glycol (PEG, in mRNA vaccines) and its derivatives including polysorbate 80 (in viral vector vaccines) have been suggested as potential culprit allergens (Table 1), possibly leading to more frequent anaphylactic reactions than to vaccines against other infectious diseases [22▪,23▪]. Recently, a group from the United Kingdom demonstrated PEG allergy in one of the first cases developing anaphylaxis to the Pfizer/BioNTech COVID-19 vaccine in the United Kingdom [24▪▪]. The patient had a previous history of anaphylaxis to other medication and milder reactions on exposure to other products suggestive of an undiagnosed PEG allergy. While PEG has not been used in vaccines before, many widely used vaccines contain polysorbate 80 [25▪].
Two of the seven vaccines presented in Table 1 are mRNA vaccines and contain PEG 2000 (Pfizer/BioNTech and Moderna). Other components are less likely to be allergenic [26▪]. Polysorbate 80 is used in most other COVID vaccines in the market, that is, AstraZeneca's Vaxzevria, Janssen's vaccine, Sputnik V and the Novavax COVID-19 Vaccine (NVX-COV2373, Novavax Inc, Gaithersburg, Maryland, USA) [1▪▪,25▪]. Polysorbates show cross-reactivity with PEG and structurally related polymers due to shared moieties [27]. However, Sinopharm, a Chinese inactivated virus vaccine, contains neither PEG nor PEG derivatives [25▪].
Polyethylene glycols and other excipients that might cause anaphylaxis
PEGs are polymers of ethylene oxide and have varying molecular weights, between 200 and 35 000 g/mol. PEGs have many names, such as Macrogol and Laureth-9, and are found in a multitude of pharmaceutical and cosmetic products. In cosmetics, the number refers to its number of ethylene oxide units whereas molecular weight is referred to in pharmaceuticals. PEG 3350 is currently contained in more than 1000 FDA-approved medications and can be found in many tablets, topical gels, laxatives and parenteral steroids [27]. PEGylation is the conjugation of PEGs to drugs, leading to prolonged half-life in plasma and less immunogenicity without compromising clinical efficacy [28,29]. Polysorbate 80 is contained in nearly 7000 FDA-approved medications and is more commonly found in vaccines, parenteral steroids, immunoglobulin replacement and tablets [30]. The formulation of PEG in the mRNA vaccines is different from the PEG used most commonly in other healthcare products, both in molecular weight and due to its coformulation as a stabilizing portion of a liposome [31–33].
Severe reports of allergies to PEG have been published [27,33,34,35▪▪,36▪▪,37,38]. A review from 2016 reports 37 cases of anaphylaxis, describing the allergen as ‘hidden’ and overlooked, that is, people have had allergic reactions with severe symptoms to several unrelated products but connection to a seemingly inert excipient is not made. The main products containing PEG that cause anaphylaxis have been oral medications, such as analgesic tablets, antibiotic tablets, antacids and laxatives, followed by injections of depot steroids. Patients with drug allergy do not normally have an adrenaline autoinjector, however, for patients with PEG allergy there is a risk of re-exposure and severe reactions, as it is not easy to avoid PEG. The patient should therefore get a written emergency plan and be issued with an adrenaline autoinjector [35▪▪,36▪▪].
Due to low awareness of PEG allergy, patients reacting to healthcare, cosmetic or hygiene products or with severe reactions to, for example, antibiotics or laxatives have been misdiagnosed with idiopathic anaphylaxis or drug allergy to specific products [35▪▪,36▪▪,37,38]. Before being diagnosed with PEG allergy, 76–80% of the patients had experienced one or more episodes of anaphylaxis requiring adrenalin treatment [27,35▪▪].
The vaccine from Moderna also contains tromethamine, an organic amine widely used in several medications for topical, enteral or parenteral administration. It is also used in cosmetics as an emulsifier. One case of anaphylaxis to tromethamine in a gadolinium-based contrast agent was reported in a 23-year-old woman; IgE-mediated trometamol allergy was detected in the patient [39]. Moreover, chlorhexidine, used to sterilize vaccine injection sites, may elicit allergic reactions. Specific IgE for chlorhexidine and skin testing may be used to establish a diagnosis [40–42].
Allergic reactions to polyethylene glycol with low and high molecular weight
Allergenicity to PEG seems to increase with increasing molecular weight and higher concentration [27,31,35▪▪,36▪▪,43▪▪,44,45,46▪▪].
Patients diagnosed with PEG allergy find it challenging to avoid products containing PEG as PEG is very widely used. Establishing PEG molecular weight thresholds would be valuable, but presently there is not enough knowledge to provide general advice on this and patients should be warned against PEGs of all molecular weights unless they have tolerated exposure to lower molecular weights [35▪▪,46▪▪].
An IgE-mediated mechanism for PEG allergy has been suggested [47]. Standardized tests to determine circulating IgE to PEGs are not commercially available, although detection of serum IgE to PEG 10 000 in patients with documented PEG-associated anaphylaxis was recently reported using a cytometric bead assay. PEG was linked to the beads with mouse anti-PEG IgG mAbs and IgE to PEG was not detected in healthy controls [43▪▪]. Positive basophil activation tests or histamine release tests have also been reported in a few patients with documented anaphylaxis reactions to products containing PEG with varying results [35▪▪,46▪▪,48–50]. However, standardized methods are lacking. Whereas an alternative mechanism underlying reactivity to PEG has also been proposed, that is, complement activation-related pseudo-allergy [51], evidence of complement activation as a mechanism for acute allergic reactions to PEG in humans is inconclusive [25▪,27,33]. Direct non-IgE-mediated activation and granulation of mast cells, via binding of the lipid nanoparticles to for example Mas-related G protein-coupled receptor X2 receptors, have also been proposed as potential mechanisms that require further investigation [52].
Investigation of patients suspected of an undiagnosed polyethylene glycol allergy or patients with severe allergic reactions to first dose of a COVID-19 vaccination
In case of immediate type reactions to the first dose of COVID-19 vaccine a blood sample taken 1.5–2 h after the reaction should be analysed for serum tryptase. Comparison with a baseline sample taken more than 24 h after resolution of symptoms should be performed. An elevation of serum tryptase more than (baseline × 1.2) + 2 supports an IgE-mediated mechanism [53]. There are no data specifically addressing the use of serum tryptase in vaccination reactions, but it is used in other parts of allergology, for example, when investigating insect venom, food allergy and perioperative allergy.
PEG and PEG derivatives such as polysorbate are testable components of the mRNA and adenovirus vector vaccines. Published data shows skin testing to be the investigation of choice to determine IgE-mediated allergy to these agents ([35▪▪,36▪▪,46▪▪]; https://www.allergyandasthmacare.com/2021/04/updated-guidance-on-risk-of-allergic-reactions-to-covid-19-vaccines.html). Although skin prick tests and intradermal tests have been used [27,36▪▪], due to the very low prevalence of PEG allergy their predictive value is still undefined, and standardized protocols are lacking. Severe allergic reactions have been described after intradermal testing with compounds containing PEG [36▪▪], and currently intradermal testing is not recommended [27]. Systemic reactions such as generalized urticaria, facial flushing and coughing, and rarely anaphylaxis, have been described during skin prick testing, especially with higher molecular weights [24▪▪,27,35▪▪,36▪▪,46▪▪].
Skin testing with undiluted vaccines might be another method of evaluating the patients, but its predictive value is unknown.
Hesselbach et al.[54] showed proportionality between molecular weight and skin prick test (SPT) reactivity. Other groups [27,46▪▪,47,55] indicate a need for testing several molecular weight PEGs. Moreover, SPT with lower molecular weight PEGs may require greater test concentrations to elicit a response [27,46▪▪,56]. Due to the risk of systemic allergic reactions on skin prick testing several groups recommend using a stepwise method, with gradually increasing molecular weights and concentrations of PEG; however, the data behind these recommendations originate from only few patients [27,36▪▪,37,46▪▪].
Wenande and Garvey [27] published a proposed algorithm for skin testing in 2016 which was very recently updated based on repeated skin testing over time in ten patients with verified PEG allergy [46▪▪]. This new algorithm is based on stepwise testing with increasing molecular weight PEGs starting with PEG 300 and stopping testing when a positive test is found. If testing on lower molecular weight PEGs is negative, increasing to using dilute solutions of PEG 20 000 has been shown to increase sensitivity of testing [46▪▪]. The study showed that skin test reactivity declines over time, so that the lower molecular weight PEGs may test negative, but higher molecular weight PEGs in increasing concentrations can be used to make the diagnosis. To asses crossreactivity to PEG derivatives skin prick testing with polysorbate 80 and poloxamer 407 is also recommended [46▪▪]. Intradermal tests should only be performed in SPT-negative patients and using diluted solutions [46▪▪].
Patients with a positive SPT against PEG or PEG derivatives are advised to avoid mRNA vaccines. The positive predictive values of SPT with PEGs and PEG derivatives is not known (https://www.allergyandasthmacare.com/2021/04/updated-guidance-on-risk-of-allergic-reactions-to-covid-19-vaccines.html), but suspected to be high based on a high number of negative controls (>350) from one centre [46▪▪].
Based on the limited evidence available in the literature, an approach to cases of probable PEG hypersensitivity suggested by the authors is described in Table 2 and for the patients who reacted to the first dose of COVID-19 vaccine in Table 3.
Table 2.
Skin prick test protocol for patients suspected to have a polyethylene glycol/polyethylene glycol derivative allergy
| Skin prick test protocol. Low index of suspicion for allergy to PEG/PEG derivatives based on Ref. [46▪▪] |
| Positive/negative control PEG 300 (100%) |
| PEG 3000 (50%) |
| PEG 6000 (50%) |
| Polysorbate 80 (20%) |
| Poloxamer 407 (10%) |
| Reading after 20 min for each SPT, all SPTs can be done the same time |
| Skin prick test protocol. High index of suspicion for allergy to PEG/PEG derivatives based on Ref. [46▪▪] | |
| If any SPT is clearly positive further testing with higher MW is not necessary. Patients with positive SPT against PEG/PEG derivatives should avoid all medicines and other products containing PEG/PEG derivatives | |
| Positive/negative control | |
| PEG 300 (100%) | |
| PEG 3000 (50%) | |
| PEG 6000 (50%) | |
| Polysorbate 80 (20%) | |
| Poloxamer 407 (10%) | |
| PEG 20 000 (0.01%) | |
| PEG 20 000 (0.1%) | |
| Stepwise method is recommended. Reading after 20 min for each step | |
MW, molecular weight; PEG, polyethylene glycol; SPT, skin prick test.
Table 3.
A suggested approach to the patients who reacted to the first dose of COVID-19 vaccine
| Mild side effects | |
| In case of mild side effects regardless of the interval after the first dose of COVID-19 vaccine such as headache, fever, tenderness in muscle, mild gastrointestinal symptoms etc. | Dose 2 of the same vaccine can be administered in any setting |
| Immediate reactions (within 4 h) | |
| Documentation of vital signs, blood pressure, saturation, chest auscultation, cutaneous symptoms, ear, nose and throat status is essential. Control tryptase | |
| Local reaction regardless of the size of the lesion | Dose 2 be administered in any setting |
| Verified anaphylaxis convincing clinical symptoms ± elevated serum tryptase | Dose 2 of the same vaccine is contraindicated. Should be referred for allergy evaluation. Dose 2 can be considered according to skin tests result and medical history in a setting with full resuscitation facilities; observation for at least 30 min is advised. Evaluate risk and benefit |
| Possible anaphylaxis or systemic symptoms such as urticaria, angioedema, severe gastrointestinal symptoms, rhinoconjunctivitis | Consider referral to allergologist/immunologist. Dose 2 can be considered according to skin tests result and medical history in a setting with full resuscitation facilities; observation for at least 30 min is advised. Evaluate risk and benefit |
| Delayed reactions (after 4 h) | |
| Documentation of vital signs, cutaneous and mucosal lesions, signs of organ failure, physical examination of the joints is advised. Skin biopsy can be considered in case of severe cutaneous/mucosal symptoms | |
| Delayed local reactions, even large exanthemas are described. Dose 2 can be administered in any setting, oral antihistamine or local steroid products can be used if itching and/or local tenderness occurs | |
| Mild systemic reactions such as urticaria, angiooedema or maculopapular exanthema can be treated with antihistamine once or twice a day (e.g. desloratadine 5–10 mg). If the symptoms resolve, dose 2 can be administered in any setting. Observation for at least 30 min is advised. Premedication with antihistamine before dose 2 (e.g. desloratadine 5–10 mg) can be considered | |
| Patients with severe delayed systemic reactions such as extensive urticaria, angiooedema, maculopapular exanthema, mucosal symptoms, arthralgia, suspected severe haematological differences, kidney or liver failure due to the vaccine should be referred to an allergologist/immunologist | |
The guidelines of the Swedish Association for Allergology for COVID-19 vaccinations are described in Table 4 as an example of a national approach.
Table 4.
Guidelines by Swedish Association for Allergology 30 April 2021
| The following guidelines apply for evaluation and routines at vaccination with the three approved vaccines Comirnaty (Pfizer) and COVID-19 Vaccine Moderna (Moderna) and COVID-19 Vaccine Vaxzevria (AstraZeneca) | |
| For all vaccinations | Remain at least 15 min after vaccination. Preparedness for anaphylaxis in place |
| For those with a severe allergic reaction to allergens such as food, insect stings, latex, contrast media, local anaesthetics or per oral medication (e.g. antibiotics) | Remain at least 30 min after vaccination. In addition to preparedness for anaphylaxis, emergency treatment should be available, including oxygen and access to peripheral venous catheterization (e.g. at a primary care centre) |
| For those with a severe allergic reaction to vaccines or other injected medication, or previous repeated anaphylaxis reactions to medications: evaluate/consider whether the person can have reacted to PEG or PEG derivative such as polysorbate or polyoxyl 35, castor oil. If no hypersensitivity exists to PEG or PEG derivatives | Consider the risk–benefit estimate for vaccination against COVID-19. Vaccination should be done at the hospital or specialist clinic with access to advanced hospital care if needed (including anaesthesia preparedness). Individual assessment of time for observation postvaccination |
| For those with a severe allergic reaction to PEG/polysorbate or polyoxyl 35 castor oil | Consider vaccination with a vaccine that does not contain PEG or PEG derivatives |
| Patient with systemic mastocytosis | Vaccination should be done at the hospital or specialist clinic with access to more advanced care if needed (including anaesthesia preparedness). Individual assessment for observation time after vaccination |
| ∗Severe allergic reaction relates to an adverse reaction in the patient's medical history that has required treatment at an emergency ward or hospitalization | |
PEG, polyethylene glycol.
CONCLUSION
COVID-19 vaccines have become available just a year after COVID-19 was first described, providing hope that vaccination can combat this pandemic. Although not yet proven, some rare adverse anaphylaxis events after vaccination may attributed to PEG and PEG derivatives. Vaccines without PEG and polysorbate, for example, Sinopharm, are already being used for mass vaccination within the European Union and could be used for those with positive skin test to PEG or its derivatives if the adverse event profile is acceptable.
In the light of increased exposure to PEGs and polysorbates in our environment and increased awareness of this rare allergy, a greater incidence of PEG hypersensitivity could be expected in the next years. It is important to investigate patients with severe allergic reactions of unknown cause to identify the rare patients with PEG allergy. Impact on daily life was shown to be better after diagnosis.
As COVID-19 can cause such severe symptoms and vaccination against COVID-19 is a key global intervention, as many people as possible need to be safely vaccinated. The underlying immunological mechanisms of severe allergic reactions to the vaccines are poorly understood and urgently need clarification.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med 2021; 384:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of the implications of early allergic reactions to the COVID-19 vaccines. The authors describe immediate and delayed reactions. Also, different polyethylene glycols (PEGs) are described as possible causes of reactions after vaccination. Different SARS-CoV-2-vaccines with these excipients are also described.
- 2.Sampson A, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006; 117:391–397. [DOI] [PubMed] [Google Scholar]
- 3.Turner PJ, Worm M, Ansotegui IJ, et al. WAO Anaphylaxis Committee. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J 2019; 12:100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance. World Allergy Organ J 2020; 13:100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rüggeberg JU, Gold MS, Bayas J-M, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5675–5684. [DOI] [PubMed] [Google Scholar]
- 6.Tizard IR. Vaccination against coronaviruses in domestic animals. Vaccine 2020; 38:5123–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020; 396:1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol 2013; 11:836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krammer F. SARS-CoV-2 vaccines in development. Nature 2020; 586:516–527. [DOI] [PubMed] [Google Scholar]
- 10.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584:457–462. [DOI] [PubMed] [Google Scholar]
- 11.Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020; 586:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US – December 14, 2020–January 18, 2021. JAMA 2021; 325:1101–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report from the CDC in the United States using the Brighton Collaboration case definition criteria for anaphylaxis. They report the incidence of anaphylaxis to be 4.7 cases/million for Pfizer/BioNTech and 2.5/million doses for Moderna.
- 13.Caminati M, Guarnieri G, Senna G. Who is really at risk for anaphylaxis due to COVID-19 vaccine? Vaccines 2021; 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol 2018; 141:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su JR, Moro PL, Ng CS, et al. Anaphylaxis after vaccination reported to the Vaccine Event Reporting System. J Allergy Clin Immunol 2019; 143:1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine – United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alnæs M, Storaas T, Sørensen M, E, Tøndell A. [COVID-19 vaccines increase the risk of anaphylaxis] [Article in Norwegian]. Tidsskr Nor Laegeforen 2021: 141. doi: 10.4045/tidsskr.21.0109. [DOI] [PubMed] [Google Scholar]
- 18.https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. [Updated 8 April 2021]. [Google Scholar]
- 19.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med 2020; 383:1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson L, Brockow K, Alm J, et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol 2017; 28:628–640. [DOI] [PubMed] [Google Scholar]
- 22▪.Cabanillas B, Akdis C, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy 2020; doi:10.1111/all.14711. [DOI] [PubMed] [Google Scholar]; One of the first publications suggesting a connection between allergic reactions to the mRNA vaccines and PEG.
- 23▪.Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth 2021; 126:e106–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first publications suggesting a connection between allergic reactions to the mRNA vaccines and PEG.
- 24▪▪.Sellaturay P, Nasser SM, Islam S, et al. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy 2021; 51:861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe the first case of a patient with a history suggestive of PEG allergy developing anaphylaxis after receiving the Pfizer/BioNTech's COVID-19 vaccine.
- 25▪.Turner PJ, Ansotegui IJ, Campbell DE, et al. Covid-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J 2021; 14:100517. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this position paper the authors describe the background to reactions to COVID-19-vaccines. They write that both the public and health care workers need reassurance that the vaccines against COVID-19 are safe.
- 26▪.Vander Leek TK, Chan ES, Connors L, et al. COVID-19 vaccine testing & administration guidance for allergists/immunologists from the Canadian Society of Allergy and Clinical Immunology (CSACI). Allergy Asthma Clin Immunol 2021; 17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review provides the first Canadian guidance to persons with suspected allergy to COVID-19 vaccines currently available.
- 27.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols; a review. Clin Exp Allergy 2016; 46:907–922. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Smith CA, Panetta JC, et al. Antibodies predict pegaspargase allergic reactions and failure of rechallenge. J Clin Oncol 2019; 37:2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahiner UM, Tolga Yavuz S, Gökce M, et al. Anaphylactic reaction to polyethylene-glycol conjugated-asparaginase: premedication and desensitization may not be sufficient. Pediatr Int 2013; 55:531–533. [DOI] [PubMed] [Google Scholar]
- 30.Caballero ML, Krantz MS, Quirce S, et al. Hidden dangers: Recognizing excipients as potential causes of drug and vaccine hypersensitivity reactions. J Allergy Clin Immunol Pract 2021; doi 10.1016/j.jaip.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone CA, Liu Y, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract 2019; 7:1533–1540.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krantz MS, Liu Y, Phillips EJ, Stone CA. Anaphylaxis to PEGylated liposomal echocardiogram contrast in a patient with IgE-mediated macrogol allergy. J Allergy Clin Immunol Pract 2020; 8:1416–1419.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 2021; 9:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox F, Khalib K, Conlon N. PEG that reaction: a case series of allergy to polyethylene glycol. J Clin Pharmacol 2021; 61:832–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪▪.Bruusgaard-Mouritsen MA, Johansen JD, Garvey LH. Clinical manifestations and impact on daily life of allergy to polyethylene glycol (PEG) in ten patients. Clin Exp Allergy 2021; 51:463–470. [DOI] [PubMed] [Google Scholar]; 10 Patients with PEG allergy are described The authors conclude that improved awareness of clinical presentation is needed and call for more clear product labelling and a standardized nomenclature to ensure the timely diagnosis of PEG allergy to prevent repeated anaphylactic reactions with severe impact on patients’ lives.
- 36▪▪.Sellaturay P, Nasser S, Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis). J Allergy Clin Immunol Pract 2020; 9:670–675. [DOI] [PubMed] [Google Scholar]; Patients with PEG allergy are described. The authors propose an investigation algorithm and highlight the risk of systemic allergic reactions to skin tests primarily intradermal test.
- 37.Brandt N, Garvey LH, Bindslev-Jensen U, et al. Three cases of anaphylaxis following injection of a depot corticosteroid with evidence of IgE sensitization to macrogols rather than the active steroid. Clin Transl Allergy 2017; 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Co-Minh HB, Demoly P, Guillot B, Raison-Peyron N. Anaphylactic shock after oral intake and contact urticaria due to polyethylene glycols. Allergy 2007; 62:92–93. [DOI] [PubMed] [Google Scholar]
- 39.Lukawska J, Mandaliya D, Chan AWE, et al. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J Allergy Clin Immunol Pract 2019; 7:1086–1087. [DOI] [PubMed] [Google Scholar]
- 40.Garvey LH, Krøigaard M, Poulsen LK, et al. IgE-mediated allergy to chlorhexidine. J Allergy Clin Immunol 2007; 120:409–415. [DOI] [PubMed] [Google Scholar]
- 41.Garvey LH, Ebo DG, Mertses P-M, et al. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy 2019; 74:1872–1884. [DOI] [PubMed] [Google Scholar]
- 42.Opstrup MS, Malling H-J, Kröigaard M, et al. Standardized testing with chlorhexidine in perioperative allergy – a large single-centre evaluation. Allergy 2014; 69:1390–1396. [DOI] [PubMed] [Google Scholar]
- 43▪▪.Zhou ZH, Stone CA, Jr, Jakubovic B, et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract 2021; 9:1731–1733.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; A cytometric bead assay was developed, enabling detection of circulating IgE to PEG 10 000 in patients with documented PEG-associated anaphylaxis, while IgE to PEG was not present in healthy controls.
- 44.Wylon K, Dölle S, Worm M. Polyethylene glycol as a cause of anaphylaxis. Allergy Asthma Clin Immunol 2016; 12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calogiuri G, Foti C, Nettis E, et al. Polyethylene glycols and polysorbates: two still neglected ingredients causing true IgE-mediated reactions. J Allergy Clin Immunol Pract 2019; 7:2509–2510. [DOI] [PubMed] [Google Scholar]
- 46▪▪.Bruusgaard-Mouritsen MA, Jensen BM, Poulsen LK, et al. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol 2021; In press. [DOI] [PubMed] [Google Scholar]; The authors have evaluated SPT to PEG with increasing molecular weight, polysorbte 80 and poloxamer in ten patients. Patients previously testing positive on SPT to PEG 3000 and/or 6000 also tested positive to PEG 20,000. Patients with longer interval since diagnosis tested positive only to higher concentrations of PEG. To avoid systemic reactions stepwise SPT is mandatory.
- 47.Wenande EC, Skov PS, Mosbech H, et al. Inhibition of polyethylene glycol-induced histamine release by monomeric ethylene and diethylene glycol: a case of probable polyethylene glycol allergy. Allergy Clin Immunol 2013; 131:1425–1427. [DOI] [PubMed] [Google Scholar]
- 48.Bommarito L, Mietta S, Nebiolo F, et al. Macrogol hypersensitivity in multiple drug allergy. Ann Allergy Asthma Immunol 2011; 107:542–543. [DOI] [PubMed] [Google Scholar]
- 49.Giangrande N, Garcia-Menaya JM, Marcos-Fernandez M, et al. Anaphylaxis due to macrogol in a laxative solution with a positive basophil activation test. Ann Allergy Asthma Immunol 2019; 123:302–304. [DOI] [PubMed] [Google Scholar]
- 50.Jover Cerdá V, Rodríguez Pacheco R, Doménech Witek J, et al. Immediate hypersensitivity to polyethylene glycols in unrelated products: when standardization in the nomenclature of the components of drugs, cosmetics, and food becomes necessary. Allergy Asthma Clin Immunol 2019; 15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozma GT, Mészáros T, Vashegyi I, et al. Pseudo-anaphylaxis to polyethylene glycol (PEG)-coated liposomes: roles of anti-PEG IgM and complement activation in a porcine model of human infusion reactions. ACS Nano 2019; 13:9315–9324. [DOI] [PubMed] [Google Scholar]
- 52.Kounis NG, Koniari I, de Gregorio C, et al. Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines 2021; 9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitte J, Sabato V, Tacquard C, et al. Use and interpretation of acute and baseline tryptase in perioperative hypersensitivity and anaphylaxis. Allergy Clin Immunol Pract 2021. [DOI] [PubMed] [Google Scholar]
- 54.Hesselbach C, Bohning W, Wettengel R. Anaphylactic shock after sucking on a throat lozenge. Dtsch Med Wochenschr 1990; 115:1397–1399. [DOI] [PubMed] [Google Scholar]
- 55.Yamasuji Y, Higashi Y, Sakanoue M, et al. A case of anaphylaxis caused by polyethylene glycol analogues. Contact Dermatitis 2013; 69:183–185. [DOI] [PubMed] [Google Scholar]
- 56.Rojas-Pérez-Ezquerra P, Crespo Quiros J, Tornero Molina P, et al. Safety of new mRNA vaccines against COVID-19 in severely allergic patients. J Investig Allergol Clin Immunol 2021; 31:180–181. [DOI] [PubMed] [Google Scholar]


