Figure 5.
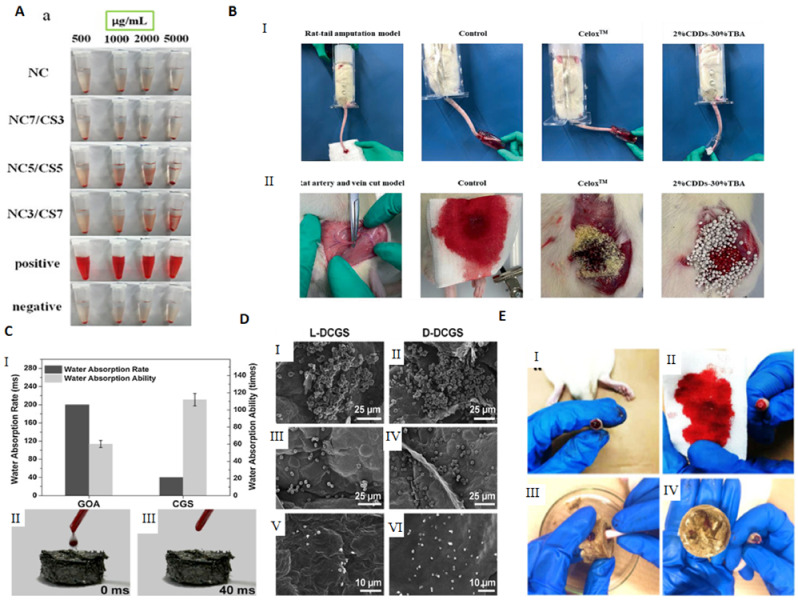
Use of bioaerogels in the treatment of wounds at the hemostasis stage: (A) photographs from hemolytic activity assay of the NC, NC7/CS3, NC5/CS5 and NC3/CS7 aerogels using sterile saline as negative control and deionized water as positive control. Notation: NC5/CS5 aerogel with 50 wt.% content of chitosan and cellulose nanofibers. Reprinted from ref [70], with permission from Elsevier. (B) In vivo hemostatic ability test and blood loss in the rat tail amputation model among the control, commercial product Celox, and chitosan/diatom-biosilica aerogel: (I) photographs of hemostatic effect and (II) blood loss in the rat tail amputation model. Adapted from ref [127] with permission from John Wiley and Sons. (C) (I) Water absorption rate and capability of cross-linked graphene sponge (CGS) and GO aerogel (GOA); (II) and (III) photographs of the blood absorption process on the CGS sample. Reprinted from ref [122], with permission from Elsevier. (D) (I,II) Images of cell morphology test of 2,3-Diaminopropionic acid cross-linked graphene sponge DCGS; (III,IV) hemocyte selective adhesion test; (V,VI) platelet selective adhesion test. Reprinted with permission from ref [125]. Copyright 2016 American Chemical Society. (E) Evaluation of the hemostatic performance of the bletilla striata polysaccharide/graphene oxide composite sponge (BGCS): (I) cutting the tail of the rat and bleeding; (II) 10 min application of gauze sponge resulted in continued bleeding; (III) BGCS was pressed on the wound; (IV) hemostasis was achieved by the BGCS. Reprinted from ref [128] with permission from Elsevier.
