Fig 8. Proposed model of PrPSc structural heterogeneity.
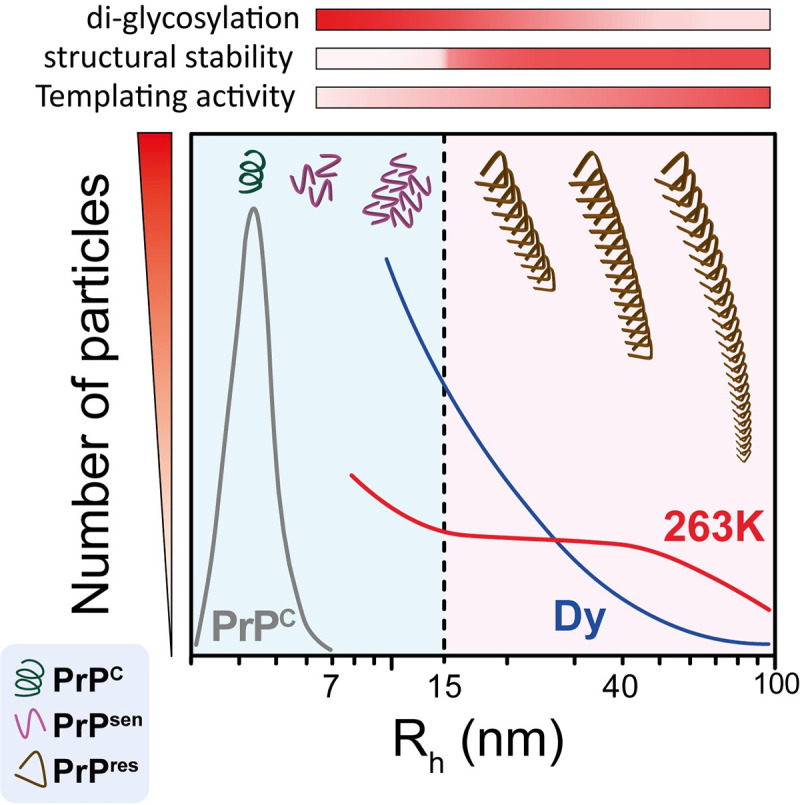
A continuum of PrPSc quaternary structures accumulate in the brain of prion-infected animals. Whereas the size of PrPSc particles is the main contributor to PrPSc heterogeneity, a major change in the core structure occurs when 15 nm Rh is reached, generating two distinctive subpopulations that define the properties of the prion strain. At this size, there is a substantial increase in resistance to proteolysis and a reduction in the incorporation of di-glycosylated PrP isoforms into the PrPSc particles. Once the mature PrPres particle is formed, the structural stability and glycosylation patterns remain constant despite further addition of PrP monomeric units. The PrPSc templating activity is also affected by the size of the particle, increasing as the particle size increases, as seen in the case of DY.
