Isoflavone-metabolizing gut bacteria ameliorate CNS autoimmunity in mice.
Abstract
The gut microbiota is a potential environmental factor that influences the development of multiple sclerosis (MS). We and others have demonstrated that patients with MS and healthy individuals have distinct gut microbiomes. However, the pathogenic relevance of these differences remains unclear. Previously, we showed that bacteria that metabolize isoflavones are less abundant in patients with MS, suggesting that isoflavone-metabolizing bacteria might provide protection against MS. Here, using a mouse model of MS, we report that an isoflavone diet provides protection against disease, which is dependent on the presence of isoflavone-metabolizing bacteria and their metabolite equol. Notably, the composition of the gut microbiome in mice fed an isoflavone diet exhibited parallels to healthy human donors, whereas the composition in those fed an isoflavone-free diet exhibited parallels to patients with MS. Collectively, our study provides evidence that dietary-induced gut microbial changes alleviate disease severity and may contribute to MS pathogenesis.
INTRODUCTION
Multiple sclerosis (MS) is a chronic neuroinflammatory disease of the central nervous system (CNS) that results in sensory, motor, and/or cognitive dysfunction (1). This is due to the complex interaction of genetic and environmental factors that trigger the activation of autoreactive T cells, leading to subsequent immune cell infiltration into the CNS, neurodegeneration, and axonal damage (2). To date, genetic influences on MS have been well characterized, such as the strong association of certain human leukocyte antigen (HLA) haplotypes with disease (3). In contrast, the role of environmental factors, which account for around 70% of disease risk, remains understudied (4, 5). Recently, the gut microbiome has emerged as a potential environmental factor that may ultimately provide critical clues to the pathogenesis and regulation of MS. Understanding the role gut microbes play in disease course may lead to possible interventions of diet, probiotics, and/or advanced combinatorial therapies for patients with MS.
Within the past decade, culture-independent microbiome profiling and sequencing technology has clearly demonstrated that the gut microbiome influences health and disease (6). Gut bacteria enable the host to harvest more energy from food by participating in the breakdown of indigestible dietary compounds into breakdown products, which can have both immunomodulatory and anti-inflammatory influences on the host immune system (7, 8). In patients with MS, certain bacteria are either enriched or depleted compared to healthy controls, indicating that gut dysbiosis occurs in these patients (9–17). As such, the gut microbiome has emerged as a potential factor that may influence the course of disease, yet it remains unclear whether differences in the abundance of specific bacteria contribute to the pathobiology of MS.
In humans, certain gut bacteria digest phytoestrogens, which are plant-based compounds that resemble estrogen. Isoflavones are a major class of phytoestrogens that are highly abundant in legumes such as soy (8). However, humans do not contain the necessary enzymes to break down isoflavones and thus rely on the gut microbiota to harvest these biologically active metabolites (18, 19). Notably, studies by our group and others found that isoflavone-metabolizing bacteria are depleted in patients with MS compared to healthy individuals, suggesting that these compounds may have anti-inflammatory properties that limit disease (9, 16). Although isoflavones are known for their antioxidant and anti-inflammatory health benefits in cardiovascular disease and cancer, the influence of these compounds on the pathogenesis and severity of MS, specifically in the context of the gut microbiome, remains elusive (18, 20).
In the present study, we demonstrate that experimental autoimmune encephalomyelitis (EAE) is suppressed in mice fed a diet supplemented with isoflavones. Furthermore, the composition of the gut microbiome in mice on an isoflavone diet exhibited parallels to that of healthy individuals, whereas the gut microbiome of those fed an isoflavone-free diet exhibited parallels to that of patients with MS. Notably, we show that certain bacteria that are absent in patients with MS are responsible for the EAE protection afforded by an isoflavone diet. Collectively, these results demonstrate that the severity/development of EAE is influenced by both diet and the resulting changes in the composition of the gut microbiome.
RESULTS
An isoflavone diet ameliorates EAE in several mouse models
Patients with MS exhibit a decreased abundance of gut bacteria that are capable of metabolizing isoflavones, suggesting that the inability to digest these compounds could contribute to or exacerbate disease (9, 16). Consistent with this, the prevalence of MS is low in countries whose citizens consume high amounts of isoflavones (10 to 30 mg/day), such as China and Japan, compared to Western countries where consumption of isoflavones is much lower (0.1 to 1 mg/day) (21). Therefore, we hypothesized that consuming a diet rich in isoflavones would provide protection against autoimmune inflammation in the CNS, thereby altering the course of EAE. To address this, we placed C57BL/6J female mice on a diet containing isoflavones or one lacking isoflavones for 6 weeks and then induced EAE using the myelin oligodendrocyte glycoprotein (MOG) epitope, MOG35-55 peptide emulsified in complete Freund’s adjuvant (CFA; MOG35-55/CFA) (Fig. 1A and table S1). Mice fed an isoflavone-free diet exhibited a robust and severe disease course, whereas this was greatly diminished in those that consumed a diet containing isoflavones (Fig. 1B). In addition, EAE clinical disease was compared with mice on a “normal feed” diet, the standard mouse chow available at the University of Iowa, which contains moderate levels of isoflavones (see Materials and Methods). The mice on normal feed exhibit an intermediate EAE phenotype between mice on an isoflavone and isoflavone-free diet (Fig. 1, A and B), although the incidence of EAE was similar in all diet groups (Fig. 1C). In addition, histological examination of spinal cord sections after immunization revealed that inflammatory cell infiltrates were increased in mice fed an isoflavone-free diet, whereas only minimal infiltrates were observed in mice that received isoflavones (Fig. 1D).
Fig. 1. An isoflavone diet ameliorates disease in multiple mouse models of EAE.
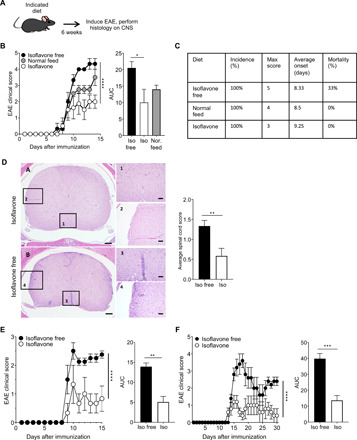
(A) Schematic of experimental design. Four- to six-week-old female mice were placed on the indicated diet for 6 weeks before immunization with MOG35-55/CFA or PLP139-151/CFA to induce EAE. Mice were euthanized 15 to 30 days after immunization, and spinal cords were harvested for analysis. (B) Comparison of mean clinical scores in C57BL/6 mice fed an isoflavone diet, isoflavone-free diet, or “normal feed” diet. Graph on the right depicts analysis of the corresponding AUC for isoflavone-free mice, isoflavone mice, and normal feed mice. (C) Various EAE metrics for the EAE clinical scores in (B). (D) Representative hematoxylin and eosin staining of spinal cord sections of mice in (B). Top, isoflavone diet; bottom, isoflavone-free diet, scored in a semiquantitative fashion. Scale bars, 200 μm and 50 μm (inset). Graph depicts the average spinal cord score, determined as described in Materials and Methods. Bars represent the mean and the standard error of data from each experimental group. Data are representative of three independent experiments with three to five mice per group. (E and F) Comparison of mean clinical scores in (E) AE°.DR2 mice or (F) SJL/J mice fed an isoflavone or isoflavone-free diet. Graph on the right depicts analysis of the corresponding AUC. Bars represent the mean and standard error of data from each experimental group. Data are representative of three independent experiments with five mice per group. P value was determined by two-way analysis of variance (ANOVA) for EAE clinical scores and Student’s t test for AUC analysis. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Population studies show that individuals with the HLA-DR2 allele exhibit an increased frequency of MS (22). Therefore, we sought to determine the impact of an isoflavone diet on AE°.DR2 transgenic female mice, which express human HLA-DR2 in a mouse major histocompatibility complex (MHC) class II negative background (23). This mouse model allows us to investigate EAE in a mouse that more accurately mimics human MS. Using the same experimental design as that for C57BL/6J mice, we observed a decreased severity of EAE in AE°.DR2 mice on an isoflavone diet compared to mice on an isoflavone-free diet, despite the fact that the response to the MOG antigen is somewhat diminished in this model (Fig. 1E). Given that 85% of patients with MS exhibit a relapsing-remitting form of disease, we also wanted to determine the effects of an isoflavone diet in this context. Therefore, we used female SJL/J mice, which develop a relapsing-remitting pattern of disease when given the myelin proteolipid protein (PLP) epitope, PLP139-151, peptide emulsified in CFA to induce EAE. Similar to the other models tested, we found that mice on an isoflavone diet also exhibit mild disease compared to mice on an isoflavone-free diet (Fig. 1F). Thus, in several models of autoimmune inflammation in the CNS, our results demonstrate that the presence of isoflavones in the diet significantly reduces the severity of EAE disease.
An isoflavone diet decreases cellular infiltration into the CNS after EAE
A hallmark of MS and EAE is proinflammatory cellular infiltration into the CNS (24). Myelin-specific CD4+ T cells that produce interferon-γ (IFN-γ), interleukin-17A (IL-17A), and/or granulocyte-macrophage colony-stimulating factor (GM-CSF) infiltrate the CNS and are critical in driving inflammation and demyelination (25). To determine the effects of an isoflavone diet on CD4+ T cell infiltration into the CNS after induction of EAE, we placed C57BL/6J female mice on either an isoflavone or isoflavone-free diet for 6 weeks, induced EAE with MOG35-55/CFA, and profiled lymphocytes in the CNS 20 days after immunization (Fig. 2A). Consistent with reduced disease severity, mice on an isoflavone diet exhibited a reduced number of infiltrating CD4+ T cells in the CNS compared to mice on an isoflavone-free diet, while the frequency of these cells was similar between mice on either diet (Fig. 2, B and C). In addition, consumption of an isoflavone diet resulted in a lower absolute number, but not frequency, of CD4+ T cells from the CNS that produced IFN-γ, IL-17A, and GM-CSF following stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Fig. 2, D to F). This is likely due to an overall decrease in immune cell infiltration into the CNS. Mice on an isoflavone diet exhibited a lower absolute number of CD45+ cellular infiltration into the CNS, with corresponding lower numbers of F4/80+ myeloid cells and a trending decrease in the numbers of CD19+ B cells (fig. S1, A to E). However, while there was a difference in proinflammatory cytokine–producing CD4+ cells within the CNS between the two groups, there was no difference in the levels of total IFN-γ and IL-17A from CNS homogenates (fig. S1F). This may be the result of differing sources of cytokine production within the CNS, as these cytokines may act differently depending on the cellular source and niche within the CNS (26). We do not find a difference in the levels of CD4+ regulatory T cells (Tregs) in the CNS of mice on either an isoflavone or isoflavone-free diet after EAE (fig. S1, G and H). These results suggest that consumption of isoflavones decreases the infiltration of proinflammatory immune cells into the CNS following induction of EAE.
Fig. 2. An isoflavone diet decreases cellular infiltration of CD4+ T cells into the CNS after EAE.
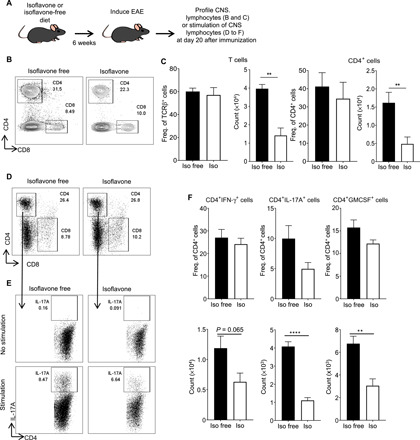
(A) Schematic of experimental design. Four- to six-week-old female mice were placed on the indicated diet for 6 weeks before immunization with MOG35-55/CFA to induce EAE. Mice were euthanized 20 days after immunization, and CNS was harvested for flow cytometric analysis of CNS leukocyte infiltration. (B) Representative flow cytometric plots of CD4+ and CD8+ T cells (gated on CD45+CD19−TCRβ+ cells) in the CNS of mice fed an isoflavone-free or isoflavone diet following the induction of EAE. Numbers represent the frequency of cells in the indicated gate. (C) The frequency and absolute number of total T cells and CD4+ T cells in the CNS following induction of EAE, as in (B). Data are representative of three independent experiments with five mice per group. (D) Representative flow cytometric analysis of CD4+ and CD8+ T cells (gated on CD45+CD19−TCRβ+ cells) in the CNS of mice fed an isoflavone or isoflavone-free diet, following the induction of EAE, and stimulated with PMA and ionomycin in the presence of brefeldin A (BFA). (E) Representative flow cytometric analysis of the IL-17A+CD4+ T cells isolated from mice, as in (D), following stimulation with PMA and ionomycin in the presence of (BFA). Numbers represent the frequency of cells in the indicated gate. Plots are gated on live CD45+TCRβ+CD4+ T cells from (D). (F) Frequency and absolute number of CD4+IFN-γ+ T cells, CD4+IL-17A+ T cells, and CD4+GMCSF+ T cells isolated from the CNS of mice in (D), following stimulation with BFA (no stimulation), or BFA and PMA/ionomycin (stimulation). Data are representative of two independent experiments with five mice per group. P value was determined by Student’s t test. **P < 0.001; ****P < 0.0001.
An isoflavone diet leads to decreased activation and proliferation of MOG-specific CD4+ T cells following induction of EAE
Following induction of EAE with myelin peptides, antigen-specific CD4+ T cells become activated, proliferate, and produce proinflammatory cytokines in the periphery before migrating to the CNS. To determine how an isoflavone diet affects this priming stage of disease, we placed C57BL/6 female mice on either an isoflavone or isoflavone-free diet for 6 weeks, induced EAE with MOG35-55/CFA, and harvested the draining lymph nodes (dLNs) before the onset of clinical symptoms (Fig. 3A). The overall number and frequency of CD4+ T cells were indistinguishable between the two diets (Fig. 3B). Similarly, the overall number and frequency of IFN-γ– and IL-17A–producing CD4+ T cells were indistinguishable between the two diets, although there was a trending decrease in the frequency of these proinflammatory cytokine–producing CD4+ T cells (Fig. 3, C and D). Unlike the CNS, where most T cells are myelin specific following induction of EAE, T cells in the dLNs may be specific for either myelin peptides or adjuvant (Mycobacterium tuberculosis) contained in the immunization emulsion. To determine whether an isoflavone diet influenced the proportion of MOG35-55 antigen-specific CD4+ T cells in the dLN, we used a MOG35-55 tetramer in conjunction with the activation marker CD44 or the proliferation marker Ki67 (27). Immunized mice on an isoflavone diet had a decreased frequency of MOG35-55-specific CD4+ T cells that were also positive for CD44 or Ki67 compared to mice on an isoflavone-free diet, suggesting that an isoflavone diet alters the proportion of autoreactive, antigen-specific CD4+ T cells (Fig. 3, E and F). To determine whether the recall response to MOG35-55 was distinct between mice on an isoflavone and isoflavone-free diet, we performed an ex vivo stimulation assay using cells from the spleen and dLN of mice fed an isoflavone or isoflavone-free diet for 6 weeks followed by immunization with MOG35-55/CFA, as described above. Seven days after immunization, cells from the spleen and dLNs were harvested and stimulated with MOG35-55 for 3 days followed by a thymidine incorporation assay. We observed that cells from the spleen and dLNs of mice on an isoflavone diet proliferated less than cells from mice on an isoflavone-free diet, suggesting that isoflavones promote an immunological environment that is less conducive to the activation of autoreactive antigen-specific CD4+ T cells (Fig. 3G). To assess whether CD4+ Tregs were limiting autoantigen-specific CD4+ T cell priming in isoflavone diet mice, we measured the levels of CD4+ Tregs in the dLNs at 7 days after immunization. However, we found no difference in the frequency or absolute number of CD4+ Tregs (fig. S1, I and J).
Fig. 3. An isoflavone diet results in lower levels of activated MOG-specific CD4+ T cells in the dLNs and spleen after EAE.
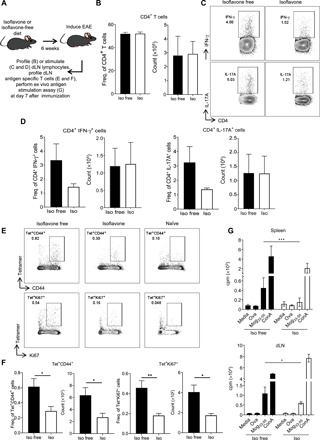
(A) Schematic of experimental design. Four- to six-week-old female mice were placed on the indicated diet for 6 weeks before immunization with MOG35-55/CFA to induce EAE. After 7 days, mice were euthanized and inguinal lymph nodes were harvested for flow cytometric analysis. (B) Frequency and absolute number of CD4+ T cells (gated on CD45+CD19−TCRβ+). (C) Representative flow cytometric plots of CD4+IFN-γ+ T cells and CD4+IL-17A+ T cells isolated from dLNs following stimulation with PMA and ionomycin in the presence of BFA. Data are representative of three independent experiments with five mice per group. (D) Frequency and absolute number of CD4+IFN-γ+ T cells and CD4+IL-17+ T cells, as in (C). (E) Representative flow cytometric plots of CD4+MOG35-55 tetramer+CD44+ T cells and CD4+MOG35-55 tetramer+Ki67+ T cells from the inguinal lymph node. Cells were gated on live TCRβ+CD4+ T cells. Data are representative of three independent experiments with five mice per group. (F) Frequency and absolute number of CD4+MOG35-55 tetramer+CD44+ T cells and CD4+MOG35-55 tetramer+Ki67+ T cells, as in (E). P value was determined by Student’s t test. (G) Proliferation of CD4+ T cells isolated from the spleen and inguinal lymph nodes of mice given an isoflavone-free or isoflavone diet. Mice were immunized as in (A), and before disease onset, cells were harvested, plated, and stimulated with MOG35-55 followed by thymidine incorporation assay. Data are representative of three independent experiments with five mice per group. P value was determined by two-way ANOVA. *P < 0.05; **P < 0.001; ***P < 0.001.
The composition of the gut microbiome is distinct between mice fed an isoflavone diet and those fed an isoflavone-free diet
Thus far, our data show that an isoflavone diet is associated with diminished antigen-specific inflammatory responses and can suppress EAE. The molecular makeup of a diet represents a major factor in determining the composition of the gut microbiota, which can affect immune cell responses (28). Therefore, we speculated that one mechanism by which isoflavones influence T cell function and suppresses disease is by altering the composition of the gut microbiome. To address this, we placed C57BL/6J female mice on either an isoflavone or isoflavone-free diet for 6 weeks, after which we performed 16S ribosomal RNA (rRNA) (V3-V4) metagenomic sequencing of bacterial DNA isolated from their feces (Fig. 4A) (29). The Chao index is a nonparametric method for estimating the number of species in a community. Using this method, we found that the diversity of bacteria within an individual host (i.e., alpha diversity) was decreased in mice on an isoflavone-free diet relative to mice on an isoflavone diet (Fig. 4B, left). This suggests that an isoflavone diet increases the species richness of the microbiome, which is associated with lower inflammation and overall better health (30). Beta diversity analysis, which compares relative bacterial abundance between two or more groups, demonstrated that the gut microbiomes are distinct between mice on an isoflavone diet and those on an isoflavone-free diet (Fig. 4B, right). Notably, specific differences in bacterial genera between mice on the two diets correlated with some bacterial differences observed between patients with MS and healthy controls (9, 14, 16, 17). Specifically, Adlercreutzia and Parabacteroides distasonis, which metabolize isoflavones, were more abundant in mice on an isoflavone diet (Fig. 4C). We also confirmed the identity of these bacteria via quantitative polymerase chain reaction (qPCR), using species-specific primers, in fecal samples from these mice (Fig. 4D). Notably, both genera were enriched in healthy individuals but depleted in patients with MS (8). Conversely, Akkermansia muciniphila was found in greater abundance in mice on an isoflavone-free diet, and this genus is commonly enriched in patients with MS compared to healthy individuals (Fig. 4C) (8). A more comprehensive analysis of differential taxonomic abundances between isoflavone and isoflavone-free mice is available in the Supplementary Materials (table S2). Given that mice on an isoflavone diet can suppress EAE, these data suggest that an isoflavone diet enables the proliferation of specific gut bacteria that can improve disease outcomes in EAE and possibly MS.
Fig. 4. An isoflavone and isoflavone-free diet alter the gut microbiome.
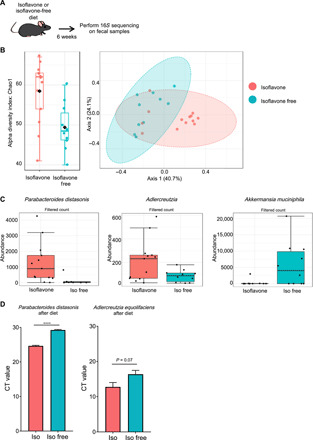
(A) Schematic of experimental design. Four- to six-week-old female mice were placed on the indicated diet for 6 weeks followed by fecal collection, bacterial DNA isolation, and 16S sequencing analysis. (B) Chao index (t test, P = 0.023655) and PCoA (permutational ANOVA, P < 0.001) plots from mice on an isoflavone or isoflavone-free diet. Each dot represents a single mouse. Isoflavone free, n = 10 mice, two cages. Isoflavone, n = 13 mice, three cages. Values are from one independent representative experiment (C). Abundance values of the P. distasonis (t test, P = 8.8102 × 10−4; FDR (false discovery rate) = 0.0039646), Adlercreutzia (t test, P = 0.014204; FDR = 0.041794), and A. muciniphila (t test, P = 2.846 × 10−4; FDR = 0.0025614) as identified by sequencing analysis in (B), in mice on an isoflavone or isoflavone-free diet. (D) Levels of Adlercreutzia equolifaciens and P. distasonis in fecal samples, measured by qPCR using species-specific primers, from mice after 6 weeks on the indicated diet. P value was determined using Student’s t test; ****P < 0.0001. Higher CT values indicate lower levels of DNA whereas lower CT values indicate higher levels of DNA.
Isoflavone-metabolizing bacteria and their metabolites are required for the suppression of EAE in mice fed an isoflavone diet
Gut bacteria metabolize dietary components leading to the production of metabolites that contribute to essential biological activity in the host. However, it is unclear whether specific bacteria, such as P. distasonis and A. equolifaciens, are required for the beneficial effects observed in mice fed an isoflavone diet. Therefore, to determine the importance of gut bacterial metabolism in isoflavone-associated EAE protection, we assessed responses to induction of EAE in mice whose gut microbiota was eliminated and reconstituted with specific genera (Fig. 5A). C57BL/6J female mice were placed on either an isoflavone or isoflavone-free diet and provided treatment with a broad-spectrum antibiotic delivered via drinking water to eliminate their gut microbiota (31, 32). Mice on both diets were subsequently treated orally with cultures of P. distasonis and A. equolifaciens for 2 weeks followed by EAE induction. We found that, in mice fed an isoflavone diet, P. distasonis and A. equolifaciens were critical for protection from EAE [Fig. 5, B (top left) and C], whereas no protection was provided in mice on either diet that received media alone (Fig. 5B, top right). In contrast, the presence of these bacteria exacerbated disease in mice fed an isoflavone-free diet (Fig. 5B, top left). We saw that the Iso + Media group had trending higher EAE clinical scores than the Iso + P. dis + A. equ group (P = 0.063) and a statistically significant difference in the area under the curve (AUC) was observed between these two groups, which represents a cumulative EAE analysis (Fig. 5C). In addition, we confirmed successful reconstitution of P. distasonis and A. equolifaciens via qPCR, using species-specific primers, in fecal samples from these mice (Fig. 5D). To confirm that isoflavone-metabolizing bacteria are specifically required for this phenomenon, we performed the same experiment by reconstituting with a non–isoflavone-metabolizing bacterium, Escherichia coli (33). We found that there was no difference in EAE between mice on either diet that received E. coli (Fig. 5B, bottom left). This suggests that isoflavone-metabolizing bacteria can uniquely protect against EAE when the host is on an isoflavone diet. Considering that A. muciniphila was increased in the gut microbiome of mice on an isoflavone-free diet, we sought to elucidate how this bacterium influences EAE in our model. Therefore, we performed the same experiment as above but reconstituted with A. muciniphila and observed that mice on an isoflavone-free diet that received A. muciniphila exhibited slightly worse EAE than isoflavone diet mice receiving the same bacterium (Fig. 5B, bottom right). This suggests that A. muciniphila may negatively contribute to EAE in mice on an isoflavone-free diet.
Fig. 5. An isoflavone diet’s ability to protect is dependent on the presence of isoflavone-metabolizing bacteria and their metabolites.
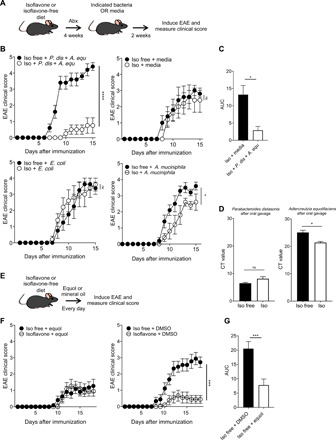
(A) Schematic of experimental design. Four- to six-week-old female mice were placed on an isoflavone diet or an isoflavone-free diet for 4 weeks while receiving broad-spectrum antibiotics in the drinking water. Subsequently, mice maintained their respective diet and were treated with either A. equolifaciens and P. distasonis, bacterial growth media, A. muciniphila, or E. coli every other day for 2 weeks followed by immunization with MOG35-55/CFA to induce EAE. (B) EAE clinical scores for mice receiving A. equolifaciens and P. distasonis (top left), bacterial growth media (top right), E. coli (bottom left), or A. muciniphila (bottom right). Data are representative of three independent experiments with five mice per group. ns, not significant. (C) Analysis of the AUC for isoflavone + media and isoflavone + P. distasonis + A. equolifaciens groups. (D) Levels of A. equolifaciens and P. distasonis in fecal samples, measured by qPCR, using species-specific primers, from mice on the indicated diet after 2 weeks of oral gavage treatment of the same bacteria, as in (A). (E) Schematic of experimental design. Four- to six-week-old female mice were placed on either an isoflavone diet or an isoflavone-free diet for 4 weeks. Mice maintained their respective diet and were orally treated with either equol or DMSO vehicle control (both resuspended in mineral oil) every day followed by immunization with MOG35-55/CFA to induce EAE. Equol and DMSO treatment were given throughout the duration of the experiment. (F) EAE clinical scores for mice receiving equol (left) or DMSO (right). Data are two combined experiments with five mice per group. (G) Analysis of the AUC for isoflavone-free + DMSO and isoflavone-free + equol. Tests with two groups were compared using a Student’s t test. P values were determined by two-way ANOVA for EAE scores. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Fecal microbiota transplantation (FMT) is a gut microbiome–based therapy currently being tested for its efficacy in treating various immune-mediated disorders (34). To determine whether an FMT from isoflavone or isoflavone-free mice influenced EAE, we treated normal feed recipients with homogenized feces from isoflavone or isoflavone-free donors (fig. S2A). We found that there was no difference in EAE clinical disease between normal feed recipients that received either isoflavone FMT or isoflavone-free FMT (fig. S2B). As the normal mouse feed has moderate levels of isoflavones, the normal feed recipients could have selected for isoflavone-metabolizing bacteria resulting in indistinguishable EAE clinical disease. To determine whether the diet of the recipient influenced the FMT, we performed a similar experiment but with isoflavone and isoflavone-free recipients (fig. S2C). We found that, albeit not statistically significant, the two isoflavone-free recipient groups had higher EAE clinical scores than the two isoflavone recipient groups (fig. S2D). This suggests that the diet of the recipient influences the therapeutic value of an FMT. Overall, these experiments suggest that in mice, isoflavone-metabolizing gut bacteria are critical for isoflavone diet–mediated protection from EAE.
Isoflavones are broken down by the Parabacteroides and Adlercreutzia genera into biologically active metabolites such as S-equol, increasing their potency (35). Therefore, we sought to determine whether treating mice with S-equol alone could ameliorate EAE. To accomplish this, we placed C57BL/6J female mice on an isoflavone or isoflavone-free diet followed immediately by administration (50 mg/kg) of S-equol or mineral oil for 4 weeks and subsequent induction of EAE (Fig. 5E). Mice received daily treatment of S-equol for the duration of the experiment. Notably, we found that treating mice on an isoflavone-free diet with S-equol ameliorated EAE, with disease severity similar to that of mice on an isoflavone diet (Fig. 5F). Moreover, we saw a significant difference in the comparison of EAE clinical scores between Iso free + DMSO (dimethyl sulfoxide) and Iso free + Equol (P < 0.001) and a statistically significant difference in the AUC between these two groups, which represents a cumulative EAE analysis (Fig. 5G). Thus, these results suggest that S-equol contributes to the disease protection afforded by an isoflavone diet.
Gut microbial-induced alterations to intestinal immunity or intestinal barrier function may be linked to changes in disease at distal organ sites (i.e., the CNS) (8). To determine how an isoflavone diet influences intestinal immunity, we analyzed the immune profile within the colonic lamina propria of C57BL/6J female mice on an isoflavone and isoflavone-free diet (fig. S3A). We observed subtle changes in the representation of certain DC populations and a potentially meaningful increase in the frequency and number of CD8+ T cells in isoflavone diet mice; however, no changes were observed in either the frequency or number of CD4+ T cells or B cells (fig. S3, B to I). To determine whether either diet influences intestinal barrier function, we performed a fluorescein isothiocyanate (FITC)–dextran intestinal permeability assay (fig. S4A). We found that mice on an isoflavone-free diet exhibit increased intestinal permeability compared to mice on an isoflavone diet at 2 hours after FITC-dextran treatment (fig. S4B). Considering that immune cells in the intestine have been shown to be involved in extraintestinal diseases, these experiments suggest that alterations in the intestinal tissue may be involved in the ability of an isoflavone diet to protect against EAE.
DISCUSSION
The human gut provides an ideal anaerobic environment for bacteria to thrive, and consequently, gut bacteria have evolved to be critical for mucosal immunity, barrier integrity, and nutrient accessibility, among other processes essential for the homeostasis of the host (8). Despite the gut microbiome being linked to MS, the mechanism by which gut bacteria predispose individuals to or offer protection from MS is unknown. Here, we demonstrate that an isoflavone diet offers protection against EAE, an animal model for MS, which is associated with a decrease in both the severity of spinal cord pathology and the number of inflammatory cells in the CNS. Our data indicate that this phenomenon is likely due to insufficient priming in the periphery after disease induction. Furthermore, the gut microbiome in mice fed an isoflavone diet has anti-inflammatory characteristics, which is comparable to a healthy gut microbiome in humans. The disease protection and anti-inflammatory phenotype associated with an isoflavone diet that we observed relied on the presence of specific bacteria that can metabolize isoflavone into S-equol, demonstrating that isoflavone-metabolizing gut bacteria that generate isoflavone metabolites provide protection against EAE. Collectively, these results suggest that metabolites of certain gut bacteria can alter CNS autoimmunity.
The finding that an isoflavone diet protects against EAE compared to a diet lacking isoflavones strongly supports the idea that environmental factors influence disease outcomes. Although the therapeutic potential of phytoestrogen compounds in EAE had been reported previously, most of the studies introduced phytoestrogens via a nonphysiological route (subcutaneously or intraperitoneally) (36). While oral phytoestrogen treatment has been reported to reduce EAE, our study demonstrates the critical role of phytoestrogen-metabolizing gut bacteria in this process (37). Our dietary model takes into account interactions among diet, gut microbiota, and immune system, thereby reflecting the physiologically relevant route/pathways through which isoflavones may protect from or ameliorate disease in patients with MS (36). Isoflavones represent a major component of the human diet, particularly in Asian countries, which have generally exhibited infrequent occurrences of MS and autoimmunity (38). Although little is known about the contributions of an isoflavone diet to MS pathogenesis, some studies have associated the consumption of soy and other legumes with reduced risk of MS or clinically isolated syndrome (CIS) (39, 40). However, as Asian cultures adopt a more Western-style diet (i.e., highly processed, high fat, excessive meat and dairy, and low fiber), they have seen an increase in the incidence in MS (41). The ability to produce equol varies between individuals, especially between individuals from different geographic locations. The frequency of so-called “equol producers” is significantly higher in Asian countries and among vegetarians than in Western countries and among nonvegetarians (42, 43). This suggests that diet has a profound impact on the ability of an individual to produce equol. However, not everyone that consumes soy can produce equol likely owing to the lack of isoflavone-metabolizing bacteria in the intestines of these so-called “non-equol producers” (44). This suggests that multiple factors, in addition to diet, contribute to isoflavone-metabolizing bacterial colonization. It would be interesting to determine whether the ability to produce equol and/or to become colonized with isoflavone-metabolizing bacteria is stunted among patients with MS. Collectively, these findings suggest that isoflavone consumption is correlated with decreased CNS autoimmunity.
The above findings suggest that nutrient intake and overall quality of diet can affect MS pathogenesis and symptomology. Consequently, most patients with MS are interested in or have implemented a dietary regimen to curb symptoms based on anecdotal evidence and recommendations from the MS patient community and/or their health care provider. Several diets have been recommended for patients with MS, including the ketogenic diet, intermittent fasting, the Mediterranean diet, gluten-free diet, the Swank diet, and the Wahls diet (45, 46). According to numerous clinical trials and epidemiological and observational studies, the efficacy of these diets in reducing symptoms and inflammatory markers are variable; some studies demonstrate clinically meaningful improvements, while other studies observing the same diet demonstrate no significant changes (47). Thus, while overall health is clearly influenced by nutritional quality, there is no evidence that a specific dietary regimen is effective in reducing the incidence of MS due to the variable results from clinical studies. The findings in our study highlight the idea that the composition of a patient’s gut microbiome is likely a critical variable in the effectiveness of dietary interventions, which has often been overlooked in prior research and clinical trials.
In humans, several studies have demonstrated that patients with MS exhibit a distinct gut microbiota compared to healthy individuals (8). While there is not a specific “MS gut microbiome signature,” some bacterial genera and species are commonly either enriched or depleted in patients with MS. For example, P. distasonis and A. equolifaciens, isoflavone-metabolizing bacteria, are reduced in patients with MS from geographically distinct cohorts (9, 16). P. distasonis, when transferred to mice, induces a Treg phenotype in the spleen and mesenteric lymph nodes (16). In addition, germ-free mice transplanted with fecal samples from healthy donors had a higher abundance of Adlercreutzia than MS fecal transplanted mice (17). Furthermore, healthy donor fecal transplanted mice developed a lower frequency of spontaneous EAE than mice transplanted with MS fecal samples (17). We found that mice on an isoflavone diet showed an increase in abundances of P. distasonis and A. equolifaciens, supporting previous reports that diet has a tremendous influence on the composition of the gut microbiota (28). In our study, we demonstrate that bacterial therapy with P. distasonis and A. equolifaciens results in markedly different clinical disease scores depending on the diet of the host. In the absence of isoflavones, these isoflavone-metabolizing bacteria may begin to metabolize host products, such as mucins, resulting in a proinflammatory state. In contrast, our results showing that equol treatment ameliorates disease, regardless of diet, strongly suggest that metabolites that are generated from gut bacteria via breakdown of dietary compounds influence the course of disease. These findings have far-reaching applications, namely, that considering the interplay between diet and gut bacteria is critical when developing dietary and gut microbiome–based therapies for MS and other diseases. Specific bacterial treatments or gut bacteria–produced metabolite treatment ameliorates or exacerbates disease, and our study expands on these findings to demonstrate the delicate interplay between diet, gut bacteria, and gut bacteria–generated metabolites and the effects of this triad on the disease. As the field becomes more aware of this connection, clinical trials are shifting to include assessment of both the diet and gut microbiome as they relate to patient outcomes.
Our study demonstrates that consuming an isoflavone diet suppresses EAE disease via gut microbial metabolism; however, the cellular and molecular pathways by which gut bacteria–generated equol suppresses disease is unknown. Specifically, mice on an isoflavone diet exhibit a lower frequency of activated myelin-specific CD4+ T cells following induction of disease, yet it is unclear how bacterial-generated equol or other metabolites dampen immune cell activity. Phytoestrogens can influence a wide range of processes, including intestinal and systemic immune responses (36). As equol, the metabolic by-product of isoflavones, is estrogenic, it is suggested that isoflavones act through estrogen receptors, particularly ERβ (36). This would correspond with the well-established idea that estrogen is protective in EAE and MS (48). Future studies determining the cells responsible for equol-dependent disease suppression are warranted. Furthermore, it is unknown if equol signals through the gut-brain axis, a bidirectional communication between the CNS and enteric nervous system, or through systemic effects, influencing the immune system directly.
Given that there are >3,000,000 genes in the gut microbiome compared to 20,000 to 25,000 protein coding genes in the entire human genome, it is expected that the gut microbiota plays a fundamental role in intestinal and systemic health (49). Our data demonstrate that diet is an environmental factor that can alter the composition of the gut microbiota, and that this has consequences on the outcome of disease. This lays the groundwork for future studies investigating the cellular and molecular pathways responsible for equol-induced EAE suppression. Ultimately, these studies will inform our use of diet and gut microbiome–based therapies as a complement to conventional disease-modifying therapies (IFN-β, glatiramer acetate, fingolimod, ocrelizumab, etc.) for the treatment of MS and other diseases.
MATERIALS AND METHODS
Mice, dietary treatment, EAE disease induction, and evaluation
C57BL/6J female mice (4 to 6 weeks old) and SJL/J female mice (4 to 6 weeks old) were purchased from the Jackson Laboratories (Bar Harbor, ME). HLA-DR15 (DRA1*0101;DB1*1501) transgenic female mice on a mouse MHC II–deficient background (AE°) (referred to here as AE°DR2 mice) have been generously provided by C. David (50). Mice were placed on either an isoflavone diet [genistein (0.24 g/kg of diet) and daidzein (0.22 g/kg of diet)] or an isoflavone-free diet (Envigo, Indianapolis, IN) ad libitum for 6 weeks. Normal feed is the standard mouse chow available at the animal facilities at the University of Iowa (Envigo 7013). For EAE studies, EAE was induced and evaluated as shown previously (51). Briefly, mice were immunized subcutaneously on day 0 on the left and right flank with 100 μg of MOG35-55 (for C57BL/6J and DR2) or 50 μg of PLP139-151 (for SJL/J) emulsified in 200 μg of CFA followed by 80 ng of pertussis toxin (PTX) intraperitoneally on days 0 and 2 (only C57BL/6 and DR2 mice received PTX). Disease severity was scored as follows: 0, no clinical symptoms; 1, loss of tail tonicity; 2, hindlimb weakness; 3, hindlimb paralysis; 4, forelimb weakness; and 5, moribund or death. All procedures were done according to the Institutional Animal Care and Use Committee guidelines at the University of Iowa.
Histology
Mice were euthanized using CO2 and intravascularly perfused using a gravity fed system with 10% neutral buffered formalin (10% NBF) via intracardiac puncture (52). Spinal cords were then emersion-fixed in 10% NBF for another 24 to 48 hours. Spinal cords were left in situ, demineralized with 14% EDTA for ~4 days, and then embedded in paraffin and routine processed. Sections (4 μm thick) were stained with hematoxylin and eosin and analyzed by a board-certified veterinary pathologist. Spinal cord sections were scored for cord pathology and meningeal inflammation. The meningeal inflammation score was a 0-to-4 scale where 0, no pathology; 1, rare, scattered, and mild meningeal inflammatory cell infiltrates; 2, mild multifocal and obvious meningeal inflammatory cell infiltrates; 3, multifocal to coalescing meningeal inflammatory cell infiltrates; and 4, marked, diffuse, and thick bands of meningeal inflammatory cell infiltrates. The spinal cord score identifies how much of the cord at that level was affected and was also a 0-to-4 scale where 0, no pathology; 1, 1 to 25% of the spinal cord is affected with pathology consistent with EAE; 2, 30 to 50% of the spinal cord is affected with pathology consistent with EAE; 3, 60 to 90% of the spinal cord is affected with pathology consistent with EAE; and 4, >90% of the spinal cord is affected with pathology consistent with EAE.
Cell isolation
Where indicated, spleens and inguinal lymph nodes were harvested, homogenized, and put into a single-cell suspension for further use. Peripheral blood was collected by retro-orbital bleeding and put into a single-cell suspension for further use. For CNS leukocyte isolation, mice were anesthetized with CO2 and quickly perfused through the left ventricle with cold phosphate-buffered saline (PBS). Brains were removed from the skull and spinal cords were flushed through the vertebral canal with cold RPMI media. To isolate immune cells from the CNS, brain and spinal cords were combined, homogenized, and isolated by Percoll gradient centrifugation (52). Following centrifugation, CNS leukocytes were collected from the interface, washed, and prepared appropriately for further use. To isolate immune cells from the colonic lamina propria, colons were harvested and digested in a collagenase/deoxyribonuclease buffer as previously described (53).
Intestinal permeability assay
To measure intestinal permeability, mice were orally treated with 4 kDa FITC-labeled dextran. Fluorescence in the serum was measured at subsequent time points and compared to preoral gavage serum (54).
Thymidine incorporation assay
To measure proliferation in the inguinal lymph nodes, cells from day 7 after immunization EAE mice were challenged with media, an irrelevant peptide OVA257-264, MOG35-55, or Concanavalin A ex vivo as previously described (55). The results are presented as counts per minute of thymidine incorporation.
Flow cytometry
Flow cytometry data were acquired on either a BD FACSCanto II or BD LSR II and analyzed with FlowJo (Ashland, OR) software. To determine surface marker expression, single-cell suspensions were incubated with monoclonal antibodies (mAbs) at 4°C for 30 min followed by fixation. For intracellular staining of cytokines/transcription factors, cells were stained for surface markers, fixed, permeabilized using FoxP3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA), and incubated with mAbs at 4°C for 30 min. The mAbs used were CD45 (30-F11, BD Biosciences), T cell receptor β (TCRβ) (H57-597, eBioscience), CD19 (6D5, BD Biosciences), CD4 (RM4-5, BD Biosciences), CD8 (53-6.7, BD Biosciences), IL-17A (TC11-18H10, eBioscience), IFN-γ (XMG1.2, eBioscience), GMCSF (granulocyte-macrophage colony-stimulating factor) (MP1-22E9, eBioscience), CD44 (IM7, BD Biosciences), Ki67 (B56, BD Biosciences), F4/80 (BM8, eBioscience), FoxP3 (FJK-16s, eBioscience), CD25 (PC61, BD Biosciences), CD11c (N418, BioLegend), I-A/I-E (M5/114.15.2, BD Biosciences), MOG tetramer [MOG38-49 GWYRSPFSRVVH, National Institutes of Health (NIH) tetramer core facility, Atlanta, GA], and Zombie Aqua (BioLegend).
PMA stimulation
Leukocytes from the CNS, spleen, or inguinal lymph node were incubated at 37°C with Cell Stimulation Cocktail (Thermo Fisher Scientific, Waltham, MA) or Protein Transport Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA) for 6 hours. Cells were stained for CD45, CD4, IFN-γ, IL-17A, and GMCSF.
Fecal collection and microbiome analysis
Microbiome analysis was done as described previously (29). Briefly, fecal pellets were collected from individual mice from each group and stored at −80°C until analysis. To reduce a cage effect, fecal samples were collected from mice in two to three cages per diet group with three to five mice in each cage. DNA was extracted using DNeasy PowerLyzer PowerSoil Kit (Qiagen). 16S rRNA V3-V4 region was amplified using PCR primers (forward 5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′; reverse 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′) and barcoded using Nextera XT Index Kit (Illumina) (29). Purified PCR products were sequenced using Illumina MiSeq.
The R-based platform Divisive Amplicon Denoising Algorithm 2 was used to trim, merge, and filter reads (56). The data were mapped against the Greengenes reference database to assign taxa designations. The Microbiome Analyst platform was used for statistical analysis and generation of figures. Data were filtered to remove low count and low variance features likely present due to contamination or error. Normalization was performed by total sum scaling. Filtered data were used for beta diversity and differential abundance. The beta diversity plot was generated using principal coordinates analysis (PCoA) with a permutational multivariate analysis of variance test to evaluate significance. Differential abundance was assessed using MetagenomeSeq, a statistical method that combines cumulative sum scaling normalization and zero-inflated Gaussian distribution to compare datasets while accounting for undersampling in high-throughput data (57). Alpha diversity analysis was performed on unfiltered data.
Gut flora depletion, colonization with bacteria, FMT, and treatment with equol
To deplete the gut flora, mice were placed on sterile water supplemented with vancomycin (0.5 g/liter), neomycin (1 g/liter), metronidazole (1 g/liter), ampicillin (1 g/liter), and Splenda (four packets/liter) for 4 weeks followed by a 2-day treatment of sterile water before treatment with bacteria (31, 32). P. distasonis ATCC (American Type Culture Collection) 8503 and Adlercreutzia equolifaciens DSM 19450 [DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH)] were ordered from their respective sources and cultured according to provided instructions. E. coli was generously provided by R. Patel from Mayo Clinic and was cultured similarly to P. distasonis and A. equolifaciens. A. muciniphila was generously provided by P. Kumar from Stony Brook University and was cultured under anaerobic conditions at 37°C using brain heart infusion (BHI) agar + 0.4% mucin or BHI broth + 0.4% mucin + l-cysteine. When indicated, mice were treated with 108 bacterial CFU (colony-forming units) every other day for 2 weeks. For FMT experiments, donor mice were placed in a sterile cage and fecal samples were collected aseptically. Feces were homogenized over a 100-μm strainer to remove large debris and diluted in 0.1% cysteine in PBS at a concentration of 1 g of feces/10 ml of 0.1% cysteine in PBS. Each recipient mouse received 150 μl of the fecal preparation. Fresh donor fecal samples were collected and processed as described every other day for 2 weeks before EAE induction. For equol treatment experiments, mice were orally treated with equol (50 mg/kg) resuspended in DMSO every day for the duration of the experiment. Equol/DMSO stocks were then diluted in mineral oil at the indicated concentration.
Quantitative polymerase chain reaction
To quantify bacterial DNA in feces, DNA was isolated and quantified as previously described (29). Gene levels were determined by qPCR using Power Sybr Green reagents (Applied Biosystems) and a QuantStudio 3 Real-Time PCR System. qPCR reactions were performed on 100 ng of bacterial DNA per sample. The following primer sets were used: P. distasonis (forward 5′ AGGGAATAAAGTGCGGGACG 3′; reverse 5′ CTTTCG TGCATCAGCGTCAG 3′) and A. equolifaciens (forward 5′ GGCGTGCTTAACACATGCAA 3′; reverse 5′ TTGCGCAAAATTCCCCACTG 3′).
Statistics
EAE groups were compared using a two-way analysis of variance (ANOVA). Tests with two groups were compared using Student’s t test. All statistics were calculated using GraphPad Prism software (La Jolla, CA). P < 0.05 was considered significant.
Acknowledgments
We thank the Karandikar, Legge, Waldschmidt, Jabbari, and Lieberman laboratories for helpful discussion. Funding: We acknowledge funding from the National Institutes of Health/NIAID (1R01AI137075), the University of Iowa Environmental Health Sciences Research Center, NIEHS/NIH (P30 ES005605), and a gift from P. Heppelmann and M. Wacek to A.K.M.; S.N.J. was supported on an institutional training grant (T32AI007485 to G. Bishop) and diversity supplement award to A.K.M. on parent 1R01AI137075. Author contributions: S.N.J. conceptualized the study, designed and performed the experiments, and wrote the manuscript. N.M.C. performed experiments and data analysis. S.K.S. and S.R.P. performed experiments. A.G. helped with data analysis. K.N.G.-C performed spinal cord pathology and histological scoring. A.K.M. conceptualized the study, designed the experiments, and edited the manuscript. All authors commented on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/28/eabd4595/DC1
REFERENCES AND NOTES
- 1.Dendrou C. A., Fugger L., Friese M. A., Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Stinissen P., Raus J., Zhang J., Autoimmune pathogenesis of multiple sclerosis: Role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit. Rev. Immunol. 17, 33–75 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Hollenbach J. A., Oksenberg J. R., The immunogenetics of multiple sclerosis: A comprehensive review. J. Autoimmun. 64, 13–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebers G. C., Bulman D. E., Sadovnick A. D., Paty D. W., Warren S., Hader W., Murray T. J., Seland T. P., Duquette P., Grey T., Nelson R., Nicolle M., Brunet D., A population-based study of multiple sclerosis in twins. N. Engl. J. Med. 315, 1638–1642 (1986). [DOI] [PubMed] [Google Scholar]
- 5.Yadav S. K., Mindur J. E., Ito K., Dhib-Jalbut S., Advances in the immunopathogenesis of multiple sclerosis. Curr. Opin. Neurol. 28, 206–219 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Kinross J. M., Darzi A. W., Nicholson J. K., Gut microbiome-host interactions in health and disease. Genome Med. 3, 14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdes A. M., Walter J., Segal E., Spector T. D., Role of the gut microbiota in nutrition and health. BMJ 361, k2179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman S. N., Shahi S. K., Mangalam A. K., The “Gut Feeling”: Breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics 15, 109–125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Chia N., Kalari K. R., Yao J. Z., Novotna M., Paz Soldan M. M., Luckey D. H., Marietta E. V., Jeraldo P. R., Chen X., Weinshenker B. G., Rodriguez M., Kantarci O. H., Nelson H., Murray J. A., Mangalam A. K., Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., Tomita A., Sato W., Kim S. W., Morita H., Hattori M., Yamamura T., Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLOS ONE 10, e0137429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tremlett H., Fadrosh D. W., Faruqi A. A., Hart J., Roalstad S., Graves J., Spencer C. M., Lynch S. V., Zamvil S. S., Waubant E.; US Network of Pediatric MS Centers , Associations between the gut microbiota and host immune markers in pediatric multiple sclerosis and controls. BMC Neurol. 16, 182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremlett H., Fadrosh D. W., Faruqi A. A., Zhu F., Hart J., Roalstad S., Graves J., Lynch S., Waubant E.; US Network of Pediatric MS Centers , Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 23, 1308–1321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremlett H., Fadrosh D. W., Faruqi A. A., Hart J., Roalstad S., Graves J., Lynch S., Waubant E.; US Network of Pediatric MS Centers , Gut microbiota composition and relapse risk in pediatric MS: A pilot study. J. Neurol. Sci. 363, 153–157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jangi S., Gandhi R., Cox L. M., Li N., von Glehn F., Yan R., Patel B., Mazzola M. A., Liu S., Glanz B. L., Cook S., Tankou S., Stuart F., Melo K., Nejad P., Smith K., Topçuolu B. D., Holden J., Kivisäkk P., Chitnis T., de Jager P. L., Quintana F. J., Gerber G. K., Bry L., Weiner H. L., Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 7, 12015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantarel B. L., Waubant E., Chehoud C., Kuczynski J., DeSantis T. Z., Warrington J., Venkatesan A., Fraser C. M., Mowry E. M., Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Invest. Med. 63, 729–734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cekanaviciute E., Yoo B. B., Runia T. F., Debelius J. W., Singh S., Nelson C. A., Kanner R., Bencosme Y., Lee Y. K., Hauser S. L., Crabtree-Hartman E., Sand I. K., Gacias M., Zhu Y., Casaccia P., Cree B. A. C., Knight R., Mazmanian S. K., Baranzini S. E., Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. U.S.A. 114, 10713–10718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berer K., Gerdes L. A., Cekanaviciute E., Jia X., Xiao L., Xia Z., Liu C., Klotz L., Stauffer U., Baranzini S. E., Kümpfel T., Hohlfeld R., Krishnamoorthy G., Wekerle H., Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. U.S.A. 114, 10719–10724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai T., Kogiso M., Soy isoflavones and immunity. J. Med. Invest. 55, 167–173 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Rietjens I., Louisse J., Beekmann K., The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 174, 1263–1280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornwell T., Cohick W., Raskin I., Dietary phytoestrogens and health. Phytochemistry 65, 995–1016 (2004). [DOI] [PubMed] [Google Scholar]
- 21.D. A. Schreihofer, Neuroprotection by dietary isoflavones and their role in cerebral ischemia, in Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease, R. R. Watson and V. R. Preedy, Eds. (Elsevier, 2015), pp. 385–394. [Google Scholar]
- 22.Barcellos L. F., Oksenberg J. R., Begovich A. B., Martin E. R., Schmidt S., Vittinghoff E., Goodin D. S., Pelletier D., Lincoln R. R., Bucher P., Swerdlin A., Pericak-Vance M. A., Haines J. L., Hauser S. L.; Multiple Sclerosis Genetics Group , HLA-DR2 dose effect on susceptibility to multiple sclerosis and influence on disease course. Am. J. Hum. Genet. 72, 710–716 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khare M., Mangalam A., Rodriguez M., David C. S., HLA DR and DQ interaction in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in HLA class II transgenic mice. J. Neuroimmunol. 169, 1–12 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Legroux L., Arbour N., Multiple sclerosis and T lymphocytes: An entangled story. J. Neuroimmune Pharmacol. 10, 528–546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatigny S., Bettelli E., Experimental autoimmune encephalomyelitis (EAE) as animal models of multiple sclerosis (MS). Cold Spring Harb. Perspect. Med. 8, a028977 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becher B., Spath S., Goverman J., Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 17, 49–59 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Sabatino J. J. Jr., Shires J., Altman J. D., Ford M. L., Evavold B. D., Loss of IFN-γ enables the expansion of autoreactive CD4+ T cells to induce experimental autoimmune encephalomyelitis by a nonencephalitogenic myelin variant antigen. J. Immunol. 180, 4451–4457 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Singh R. K., Chang H. W., Yan D., Lee K. M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T. H., Bhutani T., Liao W., Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15, 73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahi S. K., Zarei K., Guseva N. V., Mangalam A. K., Microbiota analysis using two-step PCR and next-generation 16S rRNA gene sequencing. J. Vis. Exp. , (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shreiner A. B., Kao J. Y., Young V. B., The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues R. R., Greer R. L., Dong X., DSouza K. N., Gurung M., Wu J. Y., Morgun A., Shulzhenko N., Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front. Microbiol. 8, 2306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abt M. C., Osborne L. C., Monticelli L. A., Doering T. A., Alenghat T., Sonnenberg G. F., Paley M. A., Antenus M., Williams K. L., Erikson J., Wherry E. J., Artis D., Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchihashi R., Sakamoto S., Kodera M., Nohara T., Kinjo J., Microbial metabolism of soy isoflavones by human intestinal bacterial strains. J. Nat. Med. 62, 456–460 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Skelly A. N., Sato Y., Kearney S., Honda K., Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 19, 305–323 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Rafii F., The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 5, 56–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cady N., Peterson S. R., Freedman S. N., Mangalam A. K., Beyond metabolism: The complex interplay between dietary phytoestrogens, gut bacteria, and cells of nervous and immune systems. Front. Neurol. 11, 150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahromi S. R., Arrefhosseini S. R., Ghaemi A., Alizadeh A., Sabetghadam F., Togha M., Effect of oral genistein administration in early and late phases of allergic encephalomyelitis. Iran. J. Basic Med. Sci. 17, 509–515 (2014). [PMC free article] [PubMed] [Google Scholar]
- 38.Yamasaki R., Kira J. I., Multiple sclerosis. Adv. Exp. Med. Biol. 1190, 217–247 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Black L. J., Rowley C., Sherriff J., Pereira G., Ponsonby A. L., Lucas R. M., A healthy dietary pattern associates with a lower risk of a first clinical diagnosis of central nervous system demyelination. Mult. Scler. 25, 1514–1525 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Jahromi S. R., Toghae M., Jahromi M. J., Aloosh M., Dietary pattern and risk of multiple sclerosis. Iran. J. Neurol. 11, 47–53 (2012). [PMC free article] [PubMed] [Google Scholar]
- 41.T. Yamamura, S. Miyake, Diet, gut flora, and multiple sclerosis: Current research and future perspectives, in Multiple Sclerosis Immunology (Springer, 2013). [Google Scholar]
- 42.Setchell K. D., Cole S. J., Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 136, 2188–2193 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Liu B., Qin L., Liu A., Uchiyama S., Ueno T., Li X., Wang P., Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. J. Epidemiol. 20, 377–384 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Setchell K. D., Borriello S. P., Hulme P., Kirk D. N., Axelson M., Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 40, 569–578 (1984). [DOI] [PubMed] [Google Scholar]
- 45.Katz Sand I., The role of diet in multiple sclerosis: Mechanistic connections and current evidence. Curr. Nutr. Rep. 7, 150–160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahls T. L., Chenard C. A., Snetselaar L. G., Review of two popular eating plans within the multiple sclerosis community: Low saturated fat and modified paleolithic. Nutrients 11, 352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans E., Levasseur V., Cross A. H., Piccio L., An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult. Scler. Relat. Disord. 36, 101393 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Spence R. D., Voskuhl R. R., Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol. 33, 105–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z., Tang H., Chen P., Xie H., Tao Y., Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct. Target. Ther. 4, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng S., Smart M., Hanson J., David C. S., Characterization of HLA DR2 and DQ8 transgenic mouse with a new engineered mouse class II deletion, which lacks all endogenous class II genes. J. Autoimmun. 21, 195–199 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Tyler A. F., Mendoza J. P., Firan M., Karandikar N. J., CD8+ T cells are required for glatiramer acetate therapy in autoimmune demyelinating disease. PLOS ONE 8, e66772 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahi S. K., Freedman S. N., Murra A. C., Zarei K., Sompallae R., Gibson-Corley K. N., Karandikar N. J., Murray J. A., Mangalam A. K., Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front. Immunol. 10, 462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weigmann B., Tubbe I., Seidel D., Nicolaev A., Becker C., Neurath M. F., Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Woting A., Blaut M., Small intestinal permeability and gut-transit time determined with low and high molecular weight fluorescein isothiocyanate-dextrans in C3H mice. Nutrients 10, 685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangalam A. K., Luo N., Luckey D., Papke L., Hubbard A., Wussow A., Smart M., Giri S., Rodriguez M., David C., Absence of IFN-γ increases brain pathology in experimental autoimmune encephalomyelitis-susceptible DRB1*0301.DQ8 HLA transgenic mice through secretion of proinflammatory cytokine IL-17 and induction of pathogenic monocytes/microglia into the central nervous system. J. Immunol. 193, 4859–4870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., Holmes S. P., DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paulson J. N., Stine O. C., Bravo H. C., Pop M., Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 10, 1200–1202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/28/eabd4595/DC1


