Fig. 1. Fibroblasts reverse migration direction upon sensing fluid shear at their leading edge, which promotes rapid entry of extracellular calcium.
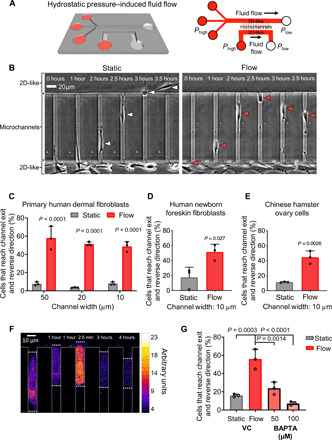
(A) Perspective view and schematic of the microfluidic device consisting of an array of parallel microchannels arranged in a ladder-like configuration and sandwiched orthogonally between two large 2D-like channels (W = 400 μm, H = 30 μm) where fluid flow is controlled. Cell migration was tracked in collagen I–coated microchannels of prescribed width (W = 10, 20, or 50 μm), height (H = 10 μm), and length (L = 200 μm). (B) In the absence of flow (static conditions), most of primary dermal fibroblasts migrate from the 2D-like seeding area into microchannels and exit to the other side into the apposing 2D-like area (white arrowheads). In contrast, 40 to 60% of these cells reverse migration direction when they reach the microchannel end and sense fluid flow in the apposing 2D-like area (red arrowheads). (C) Percentage of primary human dermal fibroblasts, (D) human newborn foreskin fibroblasts, or (E) CHO migrating cells that reach the end of the microchannels and then reverse their migration direction to remain in the microchannels under static or flow (0.5 dyne/cm2) conditions in the 2D-like regions. (F) Use of Fluo-4 Direct to visualize intracellular calcium in a migrating dermal fibroblast cell as it reaches the end of a microchannel where fluid flow is present. Scale bar, 10 μm. (G) Percentage of BAPTA- or vehicle control (VC)–treated cells that reverse migration direction in the presence of shear flow (0.5 dyne/cm2). Data represent the means ± SD from three independent experiments. Statistical comparisons were made between static and flow using two-way analysis of variance (ANOVA) followed by Sidak’s multiple comparisons test (C) or Student’s t test (D and E) or one-way ANOVA followed by Tukey’s post hoc test (G). See also fig. S1.
