Figure 2.
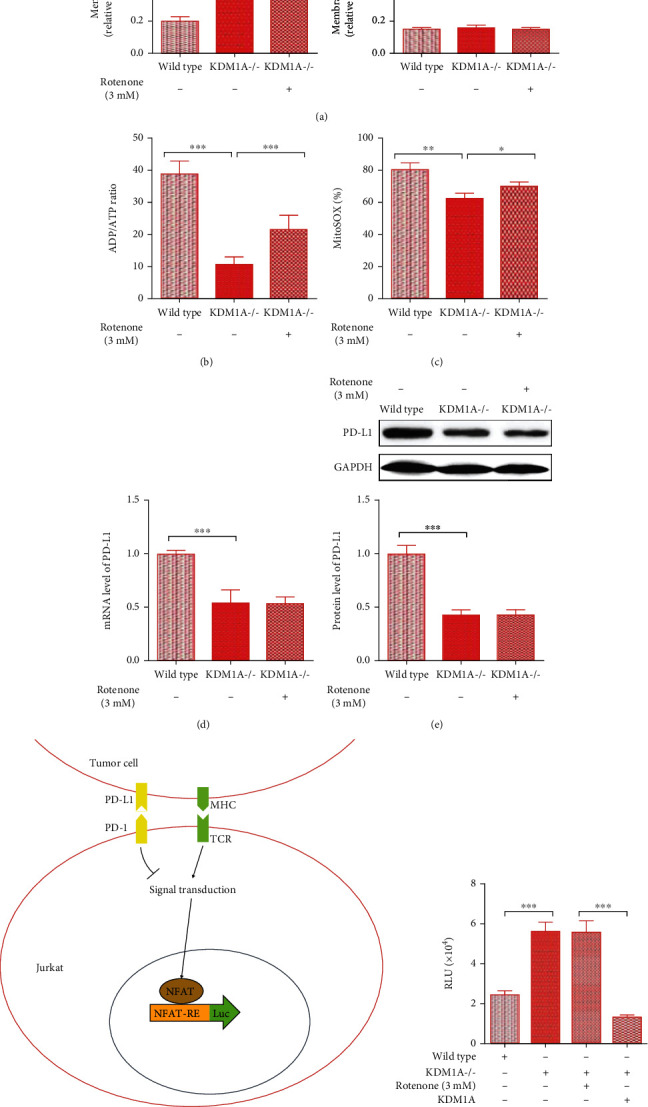
Mitochondrial dysfunction caused by elevated KDM1A does not affect the high level of PD-L1 in HCC cells. (a–c) The relative ratio of the JC-10 FL590/FL525 fluorescence intensity for mitochondrial membrane potential analysis (a), ATP/ADP analysis to determine ATP synthesis efficiency (b), and MitoSOX Red analysis to determine the mitochondrial ROS level (c) in wild-type SMMC7721 cells, SMMC7721KDM1A-/- cells, and SMMC7721KDM1A-/- cells with 3 mM rotenone treatment. (d, e) mRNA expression (d) and protein expression (e) of KDM1A and PD-L1 in the abovementioned groups. (f) Scheme of the principle of the RGA experiment. Jurkat cells have a TCR, which is responsible for recognizing tumor cells and activating the NFAT pathway. The strength of T cell-mediated antitumor immunity, which could be inhibited by the binding of PD-1 and PD-L1, is displayed by the luc2P/NFAT-RE elements in the engineered Jurkat cells. (g) The results of RGA reflecting the strength of T cell-mediated antitumor immunity in wild-type SMMC7721 cells, SMMC7721KDM1A-/- cells, SMMC7721KDM1A-/- cells treated with 3 mM rotenone, and SMMC7721KDM1A-/- cells overexpressing KDM1A. The data are presented as the means ± SD. n = 3 experiments in (a–g). ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.01.
