Abstract
To maintain their quality of life and avoid hospitalization and early mortality, patients with heart failure must recognize and respond to symptoms of exacerbation. A promising method for engaging patients in their self-care is through mobile health applications (mHealth apps). However, for mHealth to have its greatest chance for improving patient outcomes, the app content must be readable, provide useful functions and be based in evidence. The study aimed to determine: (1) readability, (2) types of functions, and (3) linkage to authoritative sources of evidence for self-care focused mHealth apps targeting heart failure patients that are available in the Apple and Google Play Stores. We systematically searched for mHealth apps targeting patients with heart failure in the Apple and Google Play Stores and applied selection criteria. Readability of randomly selected informational paragraphs were determined using Flesch–Kincaid grade level test tool in Microsoft Word. Ten mHealth apps met our criteria. Only one had a reading grade level at or below the recommended 6th grade reading level (average 9.35). The most common functions were tracking, clinical data feedback, and non-data-based reminders and alerts. Only three had statements that clearly linked the mHealth app content to trustworthy, evidence-based sources. Only two had interoperability with the electronic health record and only one had a communication feature with clinicians. Future mHealth designs that are tailored to patients’ literacy level and have advanced functions may hold greater potential for improving patient outcomes.
Keywords: heart failure, literacy, mHealth, self-care
1 |. INTRODUCTION
Over 6 million patients in the United States have heart failure (Benjamin et al., 2017), a syndrome where the heart is unable to pump enough blood to meet the body's needs, causing symptoms such as fatigue, shortness of breath, and fluid retention (American Heart Association, 2020). The disease makes it difficult for patients to perform everyday activities and contributes to high stress (Dickens et al., 2019), poor quality of life (Baert et al., 2018; Salyer et al., 2019), and early mortality (Dharmarajan & Rich, 2017; Taylor et al., 2017). It is also one of the most costly diseases worldwide with high utilization of health care services including emergency and in-patient hospitalization (Kilgore et al., 2017; Shafie et al., 2018). Self-care behaviors in patients with heart failure are critically important to patient outcomes and are associated with decreased health care utilization, improved quality of life and a decrease in early mortality (Boyde et al., 2018; Buck et al., 2012; Lee et al., 2011).
1.1 |. Self-care and heart failure
Self-care for patients with heart failure is needed in three principal ways: (1) maintenance or treatment adherence, (2) symptom perception or recognizing symptoms, and (3) management or the response to symptoms when they occur (Riegel & Dickson, 2008; Riegel et al., 2016). This includes medication management, dietary restrictions, exercise, smoking cessation, monitoring for early signs of fluid retention, and symptom tracking (Boyde et al., 2017; Moser et al., 2012). Accurately reporting information about their self-care behaviors (e.g., medication adherence), symptom experiences and day-to-day changes over time is critical in helping providers make optimal clinical decisions that can improve their patients’ quality of life and reduce the need for hospitalization.
1.2 |. Cognitive factors impacting heart failure self-care
Unfortunately, there are a variety of cognitive factors in patients with heart failure making self-care difficult. In particular, diminished cognition impacts from 25% to 79% of adults with heart failure (Hajduk et al., 2013; Harkness et al., 2014; Riegel et al., 2009; Vellone et al., 2020). This includes deficits in concentration, short-term memory, and problem solving. Compounding these deficits is the fact that many patients with heart failure also have low health literacy (Dickens, Lambert, Cromwell, et al., 2013; Riegel et al., 2009), defined as a person's ability and knowledge to make informed health care decisions (Dickens & Piano, 2013). Across many disease conditions, including heart failure, low health literacy is associated with a variety of outcomes including: increased hospitalizations, greater utilization of emergency departments, poorer ability to take medication correctly, and mortality (Berkman et al., 2011; Moser et al., 2015; Persell et al., 2020). Distinct from health literacy, low literacy, defined as “under-standing, evaluating, using and engaging with written texts to participate in society, to achieve one's goals, and to develop one's knowledge and potential” (Centers for Disease Control, 2019, para.3), is also present in over 35% of patients with heart failure (Wu et al., 2013). Low literacy in patients with heart failure is associated with increased risk for hospitalization (Wu et al., 2013).
1.3 |. Mhealth apps for heart failure self-care
Evidence demonstrates that traditional printed patient educational materials are not effective for long-term patient engagement with self-care (Alberti & Nannini, 2013; Dickson & Riegel, 2009; Unaka et al., 2017). One promising method for engaging patients in their heart failure self-care is mHealth apps (Kitsiou et al., 2019). mHealth apps, mobile applications used on telephones or electronic tablets, can simplify symptom tracking, remind and motivate patients about specific self-care activities, and store data that can improve the accuracy of communication with their providers. Well-designed health information that is understandable to patients has the potential to improve patients’ decision making, however, the evidence of the effectiveness of mHealth apps in patients with heart failure is inconsistent (Athilingam & Jenkins, 2018; Cajita et al., 2017). Given the cognitive barriers present in many patients with heart failure and the number of necessary self-care activities, for mHealth apps to have its greatest chance of improving patient outcomes, the app content must be easy to read, provide useful functions, and clearly link to trustworthy sources of clinical evidence. Other researchers have evaluated mHealth apps that focus on heart failure, but gaps in knowledge remain (Athilingam & Jenkins, 2018; Cajita, Gleason, et al., 2016; Masterson Creber et al., 2016). In particular, some of the reviews in the scholarly literature omit mHealth apps that are available in the app stores but have not been published in the scholarly literature (Athilingam & Jenkins, 2018; Cajita, Gleason, et al., 2016). In addition, we were unable to identify a scholarly review that included an assessment of mHealth apps content readability, a feature that can make a large difference in a patient’s ability to understand and apply the information from the mHealth app.
1.4 |. Objectives
The objectives of this review were to determine: (1) readability, (2) types of functions and (3) linkage to authoritative sources of evidence for self-care focused mHealth apps targeting heart failure available in the Apple and Google Play Stores.
1.5 |. Theoretical framework
Our review was guided by the technology acceptance model (TAM). The TAM model posits that intention to use a technology is influenced by the technology's ease of use and usefulness (Davis et al., 1989) The model, developed more than a quarter of a century ago, is widely regarded as one of the most valid models, frameworks, or theories used to predict why individuals accept or fail to use specific technologies (Chen et al., 2011). For this review, we operationalized ease of use as the readability level and usefulness by the types of functions and clear linkages to authoritative sources of evidence somewhere in the mHealth apps content. Our future work will examine ease of use and usability from the user's (patients with heart failure) perspective.
2 |. METHODS
2.1 |. Search criteria
In August of 2019, we identified mHealth apps targeting patients with heart failure available in Apple's App Store (iOS) and Google Play Store (Android). In a series of individual searches, our search criteria used the keywords “heart failure,” “hf,” “heart,” “heart failure app,” and “heart failure application.” The results of the searches were entered in Excel spreadsheets and duplicates were removed.
2.2 |. App selection criteria
Similar to PRISMA guidelines (Moher et al., 2010) to identify articles for Systematic Reviews and Meta-Analyses, we identified, screened, and selected mHealth apps by systematically applying prespecified selection criteria. Our exclusion criteria were mHealth apps that were non-English language based or not: heart failure specific, education and self-care focused, or targeting an adult audience. We also excluded mHealth apps that we were unable to create an account, locate, or required use of an outdated operating system.
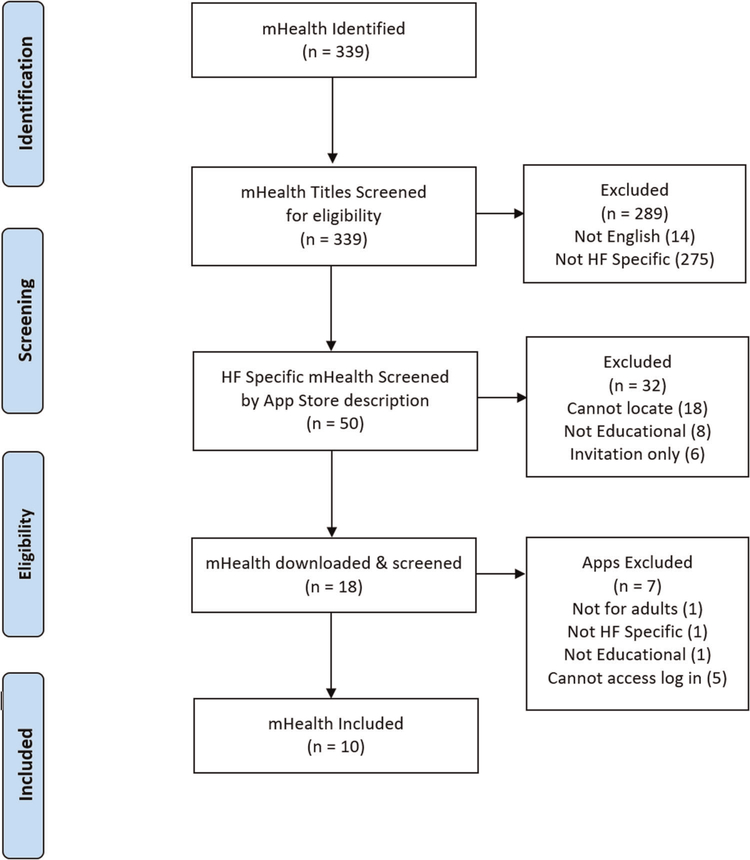
We applied the criteria in three stages: (1) mHealth apps titles, (2) App Store descriptions of the mHealth apps, and (3) fully downloaded mHealth apps (see Figure 1). To promote reliability of exclusion decisions, we used independent double coding with two authors of a subset of mHealth titles and App Store descriptions. For title exclusions (n = 339), we used a sample of 5% of the titles and achieved a 100% match in exclusion decisions between two authors in the first round. For the App Store description exclusions (n = 50), because 5% of the sample would only have been 3 mHealth app descriptions, we used a sample of 14% and achieved 100% agreement by round 2 between two authors. For the 18 downloaded mHealth apps, all were independently double coded by two authors who discussed any differences until consensus was achieved.
FIGURE 1.
mHealth apps inclusion process
2.3 |. Data extraction
We downloaded, purchased (if necessary), and registered for each of the mHealth apps that met our selection criteria on either the iPhone or Android mobile telephones. We undertook different methods to determine the readability, functionality, and evidence base linkages of the mHealth apps sample.
2.4 |. Readability
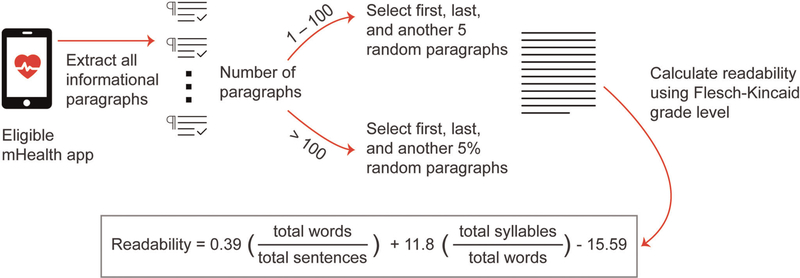
We subdivided the content from each mHealth app into informational paragraphs. For the purposes of this study to promote reliability, we defined informational paragraphs as lines of text with ≥1 sentence that began with an indent or bullet or were separated by a space. We excluded content that provided technical guidance or instructions and privacy information.
Next, we counted the number of informational paragraphs per app. To determine the readability, we selected the first and last informational paragraph in each mHealth app as well as a random selection of additional paragraphs. For mHealth apps with 1–100 informational paragraphs, we randomly sampled five of the informational paragraphs. For mHealth apps with >100 informational paragraphs, we sampled 5%, with a preset minimum of five informational paragraphs sampled per mHealth apps. We then dictated each of the selected informational paragraphs using the Dictate feature in Microsoft® Word for Office 365 ProPlus to extract the information paragraphs into Microsoft® Word. Readability grade levels were determined using Flesch–Kincaid grade level test (Kincaid et al., 1975) tool in Microsoft® Word. An overview of this process is shown in Figure 2.
FIGURE 2.
Readability assessment process
2.5 |. Functions
Beginning with functions identified by Roth (2013): information/education, diagnostic, control, and adapters, we used content analysis procedures (Hsieh & Shannon, 2005) to identify functions present in the downloaded mHealth apps. As new functions appeared, we iteratively added functions, revised function names to clarify the meaning, and rereviewed previously reviewed mHealth apps using the additional functions. The final list of functions and definitions appear in Table 1. Similar to the process used for app selection, two authors independently assessed functions for each mHealth app (SC, MG), refining definitions as needed until 100% agreement was attained.
TABLE 1.
mHealth apps functions defined
| Functions | Definition |
|---|---|
| Education | Provides information to understand health conditions |
| Diagnostic | Computes a health condition using data without human intervention |
| Remote monitoring | Allows monitoring of patient data in real-time by a care provider. |
| Tracking | Allows record-keeping of self-care behaviors (e.g. medication management, exercise), symptoms (e.g. shortness of breath, edema), and physiologic indicators (e.g. vital signs) |
| Communication | Allows sharing of information to others (e.g. clinicians, care takers, other mHealth apps users) and/or to the clinical electronic health record |
| Connect to wearables | Allows record-keeping from wearable sensors (e.g. pedometers, fit bit, smart watch) |
| Clinical Data feedback | Provides algorithmically derived feedback on patient data within the mHealth apps |
| Non-data-based reminders | Provides generic advice not based on patient data (e.g. remember not to add salt to your meals) |
| Appointments Records | Allows recording of appointments with health care providers. |
2.6 |. Evidence based
We examined each mHealth app for the presence of a statement about where the information on the mHealth apps was derived. We scored an mHealth apps to be evidence based if it included a statement referencing a well-known health organization (e.g., American Heart Association), published practice guideline, or scholarly publication from where the information was derived.
3 |. RESULTS
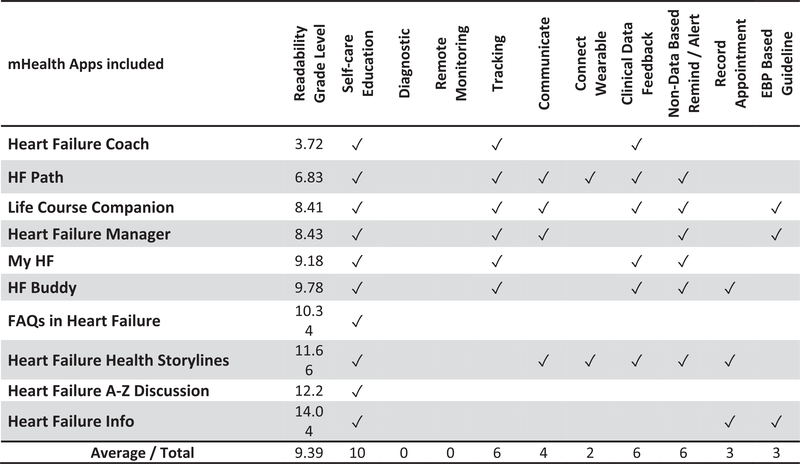
Our search methods yielded 339 unique mHealth apps. After applying our selection criteria 10 mHealth apps were included. Readability levels, identified functions, and existence of a clear source of evidence-based information is shown in Figure 3.
FIGURE 3.
Readability and functions matrix
3.1 |. Readability
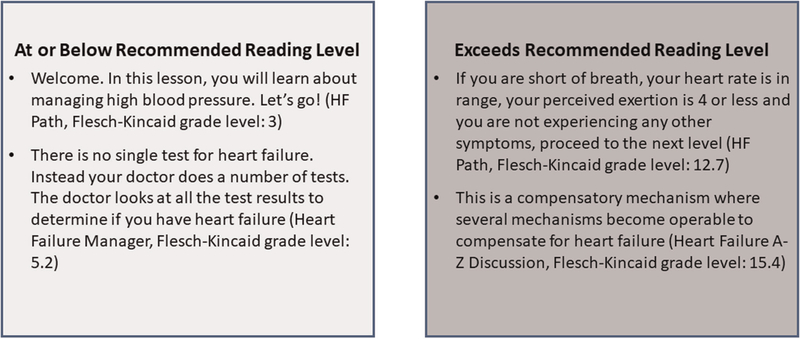
The number of informational paragraphs contained in each mHealth app ranged from 5 to 250 (avg. 85). Readability grade levels ranged from 3.72 to 14.04 (avg. 9.39). Representative examples of content at or below and above the recommended reading level from our sample are shown in Figure 4. In addition to the variability in reading levels between mHealth apps, there were also wide variability of reading levels within the mHealth app themselves. Two mHealth apps (HF Buddy and HF Path) having nearly or above a 10-year range in reading levels for their contents’ informational paragraphs (See Table 2).
FIGURE 4.
Examples of mHealth content at or below and above recommending reading level
TABLE 2.
Number of information paragraphs per mHealth app and reading grade level descriptive statistics
| Reading level |
||||
|---|---|---|---|---|
| Paragraphs | Ave | SD | Range | |
| Heart failure coach | 5 | 3.72 | 2.12 | 0.7–6.7 |
| HF Path | 250 | 6.83 | 2.69 | 2.8–12.7 |
| Life course companion | 130 | 8.41 | 2.49 | 4.1–10.8 |
| Heart failure manager | 133 | 8.43 | 2.76 | 4–12.5 |
| My HF | 69 | 9.18 | 2.24 | 5.2–10.5 |
| HF buddy | 90 | 9.78 | 5.1 | 4.8–17 |
| FAQs in heart failure | 49 | 10.34 | 2.56 | 8–14.8 |
| Heart failure health storylines | 13 | 10.34 | 2.86 | 9.2–15.5 |
| Heart failure A–Z discussion | 67 | 11.24 | 2.16 | 9.5–15.4 |
| Heart failure info | 87 | 14.04 | 2.16 | 9–15.9 |
3.2 |. Functions
To be eligible, all mHealth apps were required to have an education function. We identified eight other functions (see Table 1) in our sample. The most common features (see Figure 3) were tracking (n = 6), clinical data feedback (n = 6), and non-data-based reminders/alerts (n = 6). Two of the mHealth apps provided no functions other than self-care education.
Of the six Mhealth apps that provided a graphing function, only three (HF Path, HF Buddy, and Heart Failure Coach) provided feedback or instructions based on the graphs. Of the four apps that provided a communication function, three focused on connecting with other users. Only two apps (Heart Failure Manager and Life Course Companion) shared the data with the electronic health record.
3.3 |. Evidence-based content
Only three of the 10 mHealth apps (Heart Failure Manager, Heart Failure Info, and Life Course Companion) included a clear link to trustworthy source of evidence located in various pages of the mHealth apps. In Heart Failure Manager, the Learn More section provided a statement that the information had been reviewed and approved by the American Heart Association. The Heart Failure Info mHealth app provided information on the Economics & Research Directions section that their information was derived from British Journal, Heart, and a 2016 Cochrane review. The Life Course Companion mHealth app stated in their Learn section that their information was gathered from several authorative sources such as the National Health System, The Heart Rhythm Society, The European Society of Cardiology, the Arrythmia Alliance, and the British National Formulary.
4 |. DISCUSSION
Despite the availability of consumer-focused mHealth apps to support self-care, we were surprised to find a small number of mHealth apps targeting patients with heart failure. Of the ones that met our criteria, only one (Heart Failure Coach) was written at or below the recommended 6th grade reading level (U.S. Department of Health & Human Services & Office of Disease Prevention & Health Promotion, 2019). Of note, this mHealth app worked like playing a game to check self-care for patients with heart failure; there were only five informational paragraphs. All of the other mHealth apps had at least one informational paragraph at or above high school reading levels. This design limitation may indicate that even in the mHealth apps with an overall lower average reading level, there is likely content present that cannot be understood, or potentially can be misinterpreted, by a large percentage of patients with heart failure.
In addition to the patient self-care education function, the mHealth apps that met our selection criteria had seven different functions. In this high technology era, we were surprised to see only three mHealth apps with a function that allowed personalized feedback. As we had hoped, reading grade level was not linked to the number of functions as three of the mHealth apps with lower than high school reading levels (HF Path, Life Course Companion, and Heart Failure Manager) had some of the highest number of total functions (n = 6). We were disappointed to see that seven of the mHealth apps did not include a statement about their source of information. However, we were pleased to see that clear linkage to a trustworthy source of evidence did not appear to increase the reading grade level as two mHealth apps with lower than high school reading level (Life Course Companion and Health Failure Manager) were linked to an evidence source.
Health literacy, an individual's ability to access, understand, and use health information, can directly impact patients’ adherence to medication and health regimen, health outcomes, and health care costs (Batterham et al., 2016; Cajita, Cajita, et al., 2016; U.S. National Library of Medicine, n.d.). Patients unable to understand, remember, or apply health information are at risk for negative health outcomes through increased mortality, emergency room visits, and hospitalizations. Essential elements in providing health literate content at a 6th grade reading level include plain language, short sentences (i.e., <20 words), brief paragraphs (i.e., ≤3 sentences), bulleted or numbered lists, and actionable content (U.S. Department of Health & Human Services & Office of Disease Prevention & Health Promotion, 2019). These recommendations are especially relevant in mHealth apps as users with lower incomes and less education are more likely to access health information via mobile phones (U.S. Department of Health & Human Services & Office of Disease Prevention & Health Promotion, 2019). However, our evaluation of heart failure apps found a mean reading level of 9.3 with only one heart failure app (Health Failure Coach) meeting the evidence-based recommendations.
Another essential element in the content of heart failure apps is the differentiation between evidence-based recommendations and anecdotal examples or testimonials. With the advancement of the internet and social media, there is a proliferation of inaccurate information available to patients that finds its way into mHealth apps. Because patients with low literacy use mobile phones to access health information, it is essential that mHealth apps assist patients in understanding the importance of content that follows evidence-based guidelines by including clear statements about the source of the evidence. In our review, less than one-third of the heart failure mHealth apps included such linkages. This alarming finding is consistent with other mHealth app evaluations (Owens et al., 2018).
Overall, the most common functions included tracking, clinical data feedback, graphs of clinical data, and reminders/alerts not based upon patient data. Three of these functions (i.e., tracking, feedback, and reminders/alerts) are components of behavior change techniques (BCTs) to promote meaningful behavior changes in patients (Michie et al., 2015). These BCTs are essential in assisting health failure patients with self-monitoring at home. However, only four of the heart failure mHealth apps included all three of these essential functions.
Other functionality findings included the limited use of personalized feedback and interoperability. Personalized feedback based upon a patient’s goals and behavior is another component of BCTs (Michie et al., 2015). Only three of the heart failure mHealth apps provided some type of personalized feedback via the app. Therefore, most mHealth apps provide generic feedback or do not provide feedback at all. Previous evaluations of heart failure mHealth apps did not assess personalized feedback (Athilingam & Jenkins, 2018; Masterson Creber et al., 2016). Furthermore, the lack of interoperability between heart failure apps and electronic health records was also notable. This translates into fragmented health data, which has the potential to negatively impact patient outcomes. These findings expose the need for evidence-based algorithms to guide personalized feedback to assist heart failure patients and interoperability between the mHealth app and the electronic health record.
Our review has similarities and differences to other evaluations of heart failure apps (Athilingam & Jenkins, 2018; Masterson Creber et al., 2016). All the reviews evaluated the types of functions in heart failure apps. However, our review was the only evaluation that included an assessment of readability and links to support the evidence-based clinical guidelines. Furthermore, the other two reviews used the mobile application rating scale (MARS) to evaluate the types of functions heart failure apps, which may indicate potential gaps in the MARS tool (Stoyanov et al., 2015). For example, MARS does not include any evaluation of tracking of clinical data, connecting with wearables, providing feedback on clinical data, or communicating with family and provider, which are important elements of BCTs. Additionally, MARS does not include an evaluation of readability or inclusion of evidence-based clinical guidelines. The MARS tool does include the question, “Is the app content (visual information, language, design) appropriate for your target audience?” (Stoyanov et al., 2015). However, the assumption is that all patients in the target audience have the same reading level. Another MARS question asks, “Is the content correct, well written, and relevant to the goal/topic of the app?” (Stoyanov et al., 2015). Again, there is an assumption that correct content is equal to evidence-based practice guidelines. While the MARS tool may provide guidance on evaluating mHealth apps, it does not address the needs of mHealth apps for managing complex health conditions such as heart failure. Our findings support other researchers’ analysis of the MARS tool (Dawson et al., 2019).
In addition to the evaluation tool used, we also noted there was some, but not complete, overlap in heart failure mHealth apps included between this review and the other two (Athilingam & Jenkins, 2018; Masterson Creber et al., 2016). Five of the mHealth apps included in this review were not included in the two earlier reviews. Although neither of the other studies evaluated readability, our review concurs with these earlier reviews that concluded that there is room for improvement in the features of mHealth apps supporting heart failure self-care.
Mobile health apps are one of the fastest expanding areas of health care with over 325 000 mHealth apps available for download (Research2Guidance, 2018). In spite of this exponential growth, there is no standardized tool or guide for evaluating this vast number of mHealth apps. As we described above, there is variation in evaluation methods. The lack of comprehensive evaluation tools or rating systems may stem from the absence of mHealth apps standards. However, this current gap creates confusion and inconsistencies in evaluating mHealth apps, which may ultimately hinder provider and patient adoption of mHealth apps. Based upon these findings, we recognize the need for standardized rating system for the evaluation of mHealth apps that incorporates readability standards to assist with patients’ understanding and links to the source to differentiate anecdotal information from evidence-based clinical guidelines.
4.1 |. Limitations
The primary limitation of this study was the limited number of informational paragraphs assessed per mHealth app. With some mHealth apps having >300 lessons, it was not feasible to evaluate each lesson. Using a randomly selected lesson partially overcomes this limitation. Future research should examine a larger sample of lessons and variability of readability within mHealth apps. In addition, the Flesch–Kincaid formula assesses readability based on the average number of syllables per word and the average number of words per sentence. It does not take format into consideration (such as information chunking) that can impact readability. We were unable to identify clear guidance about the percent of content needed for double coders of app store reviews, the use of 5% double coding of the titles is less than the 10%–20% data sample commonly used for qualitative data double coding. We acknowledge that some of the information contained in the mHealth apps may be evidence based but did not include a statement of their evidence source. However, with the ease that developers can develop and market mHealth apps that are not linked to clinical evidence, we believe mHealth apps should clearly indicate a trustworthy source of evidence. Finally, we note that the vendors of mobile phones vary by country. If examining mHealth apps directly available to consumers internationally or in sub-cultures within a country, it is important to consider additional app stores that may be used in that setting (e.g., Huawei App Gallery).
5 |. CONCLUSION
Nurses play an essential role in patient education and in promoting a patient’s self-care. In this age of proliferating health information technologies, nurses should be aware of the variety of options in mHealth apps that are available directly to patients in app stores. Nurses can guide patients to use tools that are derived from trustworthy sources, have the needed functions for each patient’s situation, and are easy to understand.
ACKNOWLEDGMENT
Drs. Dunn Lopez, Chattopadhyay and Ms. Habibi are supported by The National Cancer Institute (R01 CA225446-01, Di Eugenio B.-PI). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute.
Funding information
National Cancer Institute, Grant/Award Number: R01 CA225446-01
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- Alberti TL, & Nannini A (2013). Patient comprehension of discharge instructions from the emergency department: A literature review. Journal of the American Academy of Nurse Practitioners, 25(4), 186–194. [DOI] [PubMed] [Google Scholar]
- American Heart Association. (2020). What is heart failure? https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure
- Athilingam P, & Jenkins B (2018). May 2). Mobile phone apps to support heart failure self-care management: Integrative review. Journal of Medical Internet Research Cardio, 2(1), e10057. 10.2196/10057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert A, De Smedt D, De Sutter J, De Bacquer D, Puddu PE, Clays E, & Pardaens S (2018). Factors associated with health-related quality of life in stable ambulatory congestive heart failure patients: Systematic review. European Journal of Preventive Cardiology, 25(5), 472–481. [DOI] [PubMed] [Google Scholar]
- Batterham RW, Hawkins M, Collins PA, Buchbinder R, & Osborne RH (2016). March). Health literacy: Applying current concepts to improve health services and reduce health inequalities. Public Health, 132, 3–12. 10.1016/j.puhe.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, Floyd J, Fornage M, Gillespie C, & Isasi C (2017). Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation, 135(10), e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, & Crotty K (2011). Low health literacy and health outcomes: An updated systematic review. Annals of Internal Medicine, 155(2), 97–107. [DOI] [PubMed] [Google Scholar]
- Boyde M, Peters R, Hwang R, Korczyk D, Ha T, & New N (2017). The self-care educational intervention for patients with heart failure: A study protocol. Journal of Cardiovascular Nursing, 32(2), 165–170. [DOI] [PubMed] [Google Scholar]
- Boyde M, Peters R, New N, Hwang R, Ha T, & Korczyk D (2018). Self-care educational intervention to reduce hospitalisations in heart failure: A randomised controlled trial. European Journal of Cardiovascular Nursing, 17(2), 178–185. [DOI] [PubMed] [Google Scholar]
- Buck HG, Lee CS, Moser DK, Albert NM, Lennie T, Bentley B, Worrall-Carter L, & Riegel B (2012). Relationship between self-care and health-related quality of life in older adults with moderate to advanced heart failure. Journal of Cardiovascular Nursing, 27(1), 8–15. [DOI] [PubMed] [Google Scholar]
- Cajita MI, Cajita TR, & Han H-R (2016). Health literacy and heart failure: A systematic review. Journal of Cardiovascular Nursing, 31(2), 121–130. 10.1097/JCN.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajita MI, Gleason KT, & Han H-R (2016). A systematic review of mHealth-based heart failure interventions. The Journal of Cardiovascular Nursing, 31(3), E10–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajita MI, Hodgson NA, Budhathoki C, & Han H-R (2017). Intention to use mHealth in older adults with heart failure. The Journal of Cardiovascular Nursing, 32(6), E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. (2019). Understanding Literacy and Numeracy. Retrieved from https://www.cdc.gov/healthliteracy/learn/UnderstandingLiteracy.html
- Chen S-C, Shing-Han L, & Chien-Yi L (2011). Recent related research in technology acceptance model: A literature review. Australian Journal of Business and Management Research, 1(9), 124–127. [Google Scholar]
- Davis F, Davis G, Morris M, & Venkatesh VJ (1989). Technology acceptance model. Management Science, 35(8), 982–1003. [Google Scholar]
- Dawson R, Felder T, Donevant S, McDonnell K, Card E, Campbell C, & Heiney S (2019). What makes a good health “app”? Identifying the strengths and limitations of existing mobile application evaluation tools. Nursing Inquiry, 27. 10.1111/nin.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan K, & Rich MW (2017). Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Failure Clinics, 13(3), 417–426. [DOI] [PubMed] [Google Scholar]
- Dickens C, Dickson VV, & Piano MR (2019). Perceived stress among patients with heart failure who have low socioeconomic status: A mixed-methods study. Journal of Cardiovascular Nursing, 34(3), E1–E8. [DOI] [PubMed] [Google Scholar]
- Dickens C, Lambert BL, Cromwell T, & Piano MR (2013). Nurse overestimation of patients' health literacy. Journal of Health Communication, 18(Suppl. 1), 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens C, & Piano MR (2013). Health literacy and nursing: An update. The American Journal of Nursing, 113(6), 52–57. [DOI] [PubMed] [Google Scholar]
- Dickson VV, & Riegel B (2009). Are we teaching what patients need to know? Building skills in heart failure self-care. Heart & Lung, 38(3), 253–261. [DOI] [PubMed] [Google Scholar]
- Hajduk AM, Lemon SC, McManus DD, Lessard DM, Gurwitz JH, Spencer FA, Goldberg RJ, & Saczynski JS (2013). Cognitive impairment and self-care in heart failure. Clinical Epidemiology, 5, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness K, Heckman GA, Akhtar-Danesh N, Demers C, Gunn E, & McKelvie RS (2014). Cognitive function and self-care management in older patients with heart failure. European Journal of Cardiovascular Nursing, 13(3), 277–284. [DOI] [PubMed] [Google Scholar]
- Hsieh H-F, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Patel HK, Kielhorn A, Maya JF, & Sharma P (2017). Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Management and Healthcare Policy, 10,63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid JP, Fishburne R, Rogers R, & Chissom BJMNAS (1975). Derivation of new readability formulas (Automated Readability Index, Fog Count, Flesch Reading Ease) for Navy enlisted personnel. Research Branch Report 8–75.
- Kitsiou S, Vatani H, Paré G, Gerber B, Buchholz SW, Kansal MM, & Masterson Creber RM (2019). Effectiveness of mobile health technology interventions on patients with heart failure: Systematic review and meta-analysis. Circulation, 140(Suppl_1), A15772–A15772. [DOI] [PubMed] [Google Scholar]
- Lee CS, Moser DK, Lennie TA, & Riegel B (2011). Event-free survival in adults with heart failure who engage in self-care management. Heart & Lung, 40(1), 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson Creber RM, Maurer MS, Reading M, Hiraldo G, Hickey KT, & Iribarren S (2016). Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the Mobile Application Rating Scale (MARS). Journal of Medical Internet Research mHealth and uHealth, 4(2), e74. 10.2196/mhealth.5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, & Hardeman W (2015). Behaviour change techniques: The development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (A suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technology Assessment, 19(99), 1–188. 10.3310/hta19990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2010). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Moser DK, Dickson V, Jaarsma T, Lee C, Stromberg A, & Riegel B (2012). Role of self-care in the patient with heart failure. Current Cardiology Reports, 14(3), 265–275. [DOI] [PubMed] [Google Scholar]
- Moser DK, Robinson S, Biddle MJ, Pelter MM, Nesbitt TS, Southard J, Cooper L, & Dracup K (2015). Health literacy predicts morbidity and mortality in rural patients with heart failure. Journal of Cardiac Failure, 21(8), 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens OL, Beer JM, Reyes LI, Gallerani DG, Myhren-Bennett AR, & McDonnell KK (2018). Mindfulness-based symptom and stress management apps for adults with chronic lung disease: Systematic search in app stores. Journal of Medical Internet Research mHealth and uHealth, 6(5), e124. Article e124. 10.2196/mhealth.9831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persell SD, Karmali KN, Lee JY, Lazar D, Brown T, Friesema EM, & Wolf MS (2020). Associations between health literacy and medication self-management among community health center patients with uncontrolled hypertension. Patient Preference and Adherence, 14, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research2Guidance. (2018). 325,000 mobile health apps available in 2017 - Android now the leading mHealth platform. Research2Guidance. Retrieved from https://research2guidance. com/325000-mobile-health-apps-available-in-2017/ [Google Scholar]
- Riegel B, & Dickson VV (2008). A situation-specific theory of heart failure self-care. Journal of Cardiovascular Nursing, 23(3), 190–196. [DOI] [PubMed] [Google Scholar]
- Riegel B, Dickson VV, & Faulkner KM (2016). The situation-specific theory of heart failure self-care: Revised and updated. Journal of Cardiovascular Nursing, 31(3), 226–235. [DOI] [PubMed] [Google Scholar]
- Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, & Lindenfeld J (2009). State of the science: Promoting self-care in persons with heart failure: A scientific statement from the American Heart Association. Circulation, 120(12), 1141–1163. [DOI] [PubMed] [Google Scholar]
- Roth VJ (2013). The mHealth conundrum: Smartphones & mobile medical apps—How much FDA medical device regulation is required. NNorth Carolina Journal of Law & Technology, 15, 359–424. [Google Scholar]
- Salyer J, Flattery M, & Lyon DE (2019). Heart failure symptom clusters and quality of life. Heart & Lung, 48(5), 366–372. [DOI] [PubMed] [Google Scholar]
- Shafie AA, Tan YP, & Ng CH (2018). Systematic review of economic burden of heart failure. Heart Failure Reviews, 23(1), 131–145. [DOI] [PubMed] [Google Scholar]
- Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, & Mani M (2015). Mobile app rating scale: A new tool for assessing the quality of health mobile apps. Journal of Medical Internet Research mHealth and uHealth, 3(1). 10.2196/mhealth.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, & Marshall T (2017). Survival following a diagnosis of heart failure in primary care. Family Practice, 34(2), 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unaka NI, Statile A, Haney J, Beck AF, Brady PW, & Jerardi KE (2017). Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions. Journal of Hospital Medicine, 12(2), 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services, & Office of Disease Prevention & Health Promotion. (2019). Health literacy online: A guide to simplifying the user experience. Retrieved from https://health.gov/our-work/health-literacy/health-literacy-online
- U.S. National Library of Medicine. (n.d.). Health literacy. National Network of Libraries of Medicine. Retrieved from https://nnlm.gov/initiatives/topics/health-literacy
- Vellone E, Chialà O, Boyne J, Klompstra L, Evangelista LS, Back M, Ben Gal T, Mårtensson J, Strömberg A, & Jaarsma T (2020). Cognitive impairment in patients with heart failure: An international study. ESC Heart Failure, 7(1), 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J-R, Holmes GM, DeWalt DA, Macabasco-O'Connell A, Bibbins-Domingo K, Ruo B, Baker DW, Schillinger D, Weinberger M, & Broucksou KA (2013). Low literacy is associated with increased risk of hospitalization and death among individuals with heart failure. Journal of General Internal Medicine, 28(9), 1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]