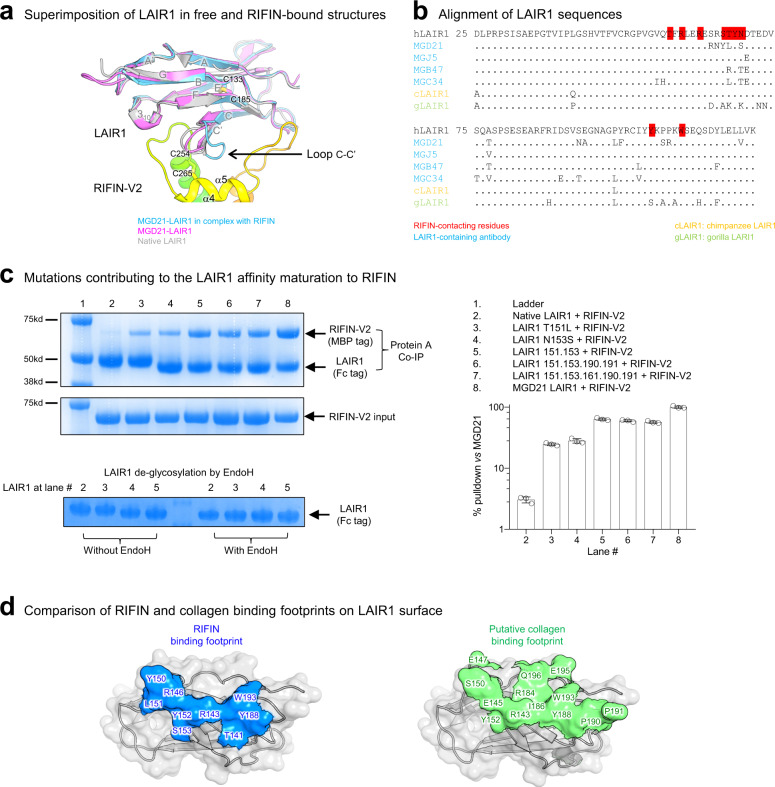
Fig. 2. Structural and sequence analysis of various LAIR1 constructs identifies RIFIN-binding essential.
a Conformational change on LAIR1 c-c’ loop to enable RIFIN binding. b Alignment of LAIR1 sequences. (MGD21, hLAIR1: native human LAIR1, MGJ5, MGB47, MGC34, gLAIR1: gorilla LAIR1). c Accumulation of critical mutations allows affinity maturation of LAIR1 to RIFIN (upper left panel: SDS-PAGE image; lower left: LAIR1 de-glycosylation by EndoH; upper right panel: SDS-PAGE sample loading list; lower right panel: Quantification of left panel by bar graph. Data are presented as mean values ± SD). n = 3 independent measurements. The SDS-PAGE runs were repeated at least twice. d RIFIN binding footprint overlapped that of collagen on LAIR1 surface. Note: LAIR1 residue numbering is based on MGD21 construct, which has 84 shift to that of native LAIR1.