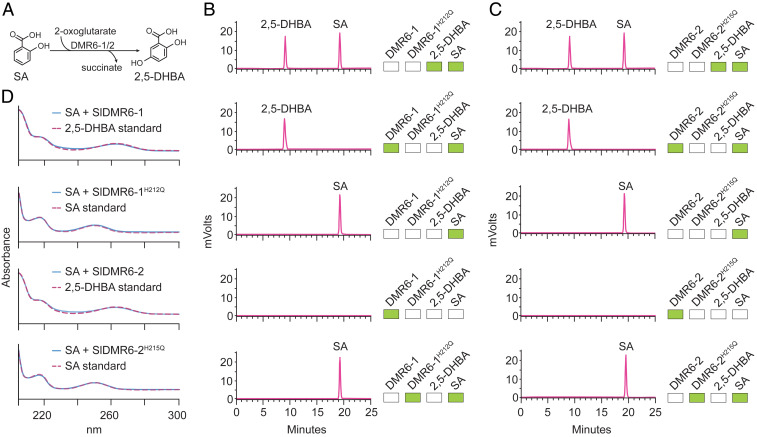
Fig. 5.
SlDMR6-1 and SlDMR6-2 catalyze the conversion of SA to 2,5-DHBA. (A) The reaction catalyzed by SlDMR6-1 and SlDMR6-2 showing the hydroxylation of SA at carbon 5 with the subsequent formation of 2,5-DHBA. (B) HPLC profile of the standards 2,5-DHBA and SA (first panel). SA is converted to 2,5-DHBA by the recombinant SlDMR6-1 protein (second panel), but not in the control reaction with no enzyme (third panel). Enzyme preparation contained no contaminants (fourth panel). Mutation of the active site of SlDMR6-1 (SlDMR6-1 H212Q) prevents the conversion of SA to 2,5-DHBA (fifth panel). (C) HPLC profile of the standards 2,5-DHBA and SA (first panel). SA is converted to 2,5-DHBA by the recombinant SlDMR6-2 protein (second panel), but not in the control reaction with no enzyme (third panel). Enzyme preparation contained no contaminants (fourth panel). Mutation of the active site of SlDMR6-2 (SlDMR6-2 H215Q) prevents the conversion of SA to 2,5-DHBA (fifth panel). The green boxes in B and C indicate the presence of that compound in the reaction mixture. (D) Comparison of the absorbance spectra of SA/2,5-DHBA standards to the products of the enzyme assays. The absorbance spectra of the enzymatic product 2,5-DHBA from B and C are identical to that of the 2,5-DHBA standards. All reactions were repeated at least three times and representative data are shown.