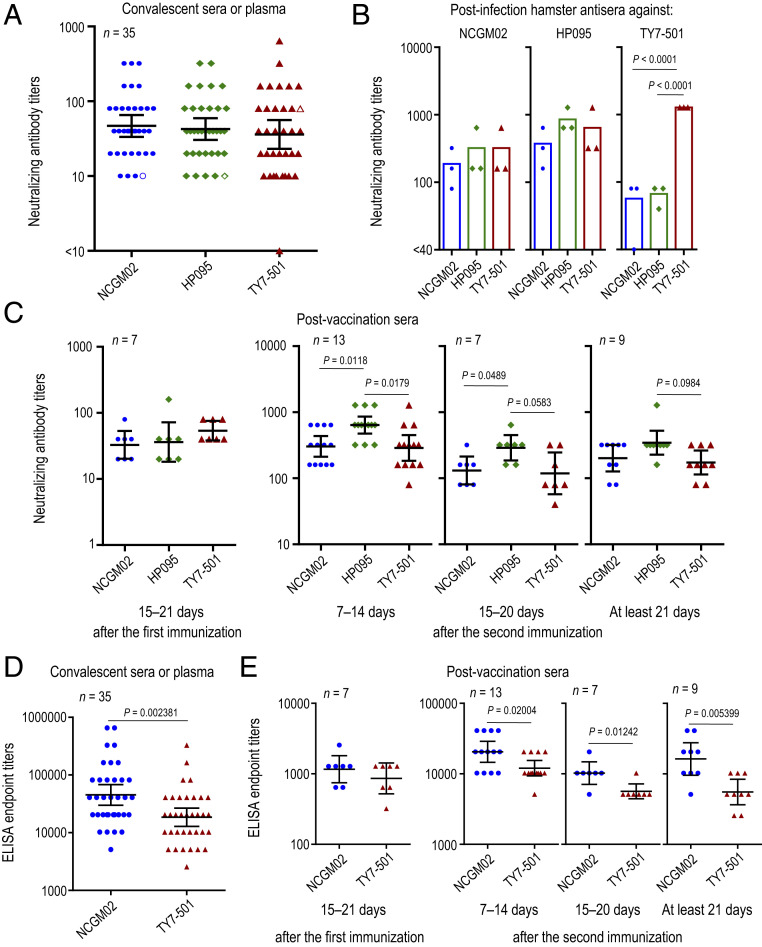
Fig. 3.
Antibody responses to the SARS-CoV-2 P.1 variant. (A) Neutralizing antibody titers of human sera or plasma obtained from recovered COVID-19 patients. Open symbols indicate the titers of serum from patient NCCo-503, who was infected with a P.1 variant. (B) Neutralizing antibody titers of postinfection hamster sera against SARS-CoV-2 variants. Syrian hamsters were intranasally inoculated with 103 PFU of TY7-501, NCGM02, or HP095. Sera were collected on day 21 postinfection. (C) Neutralizing antibody titers in sera from BNT162b2 vaccinees. P values were calculated by using a one-way ANOVA, followed by Tukey’s post hoc test. (D and E) ELISA titers of antibodies against the RBD from TY7-501 or NCGM02 (D) in convalescent sera or plasma and (E) in sera from BNT162b2 vaccinees. P values were calculated by using a two-tailed unpaired Wilcoxon’s rank sum test with a continuity correction. Each dot represents data from one subject. Geometric mean titer and 95% CIs are shown.