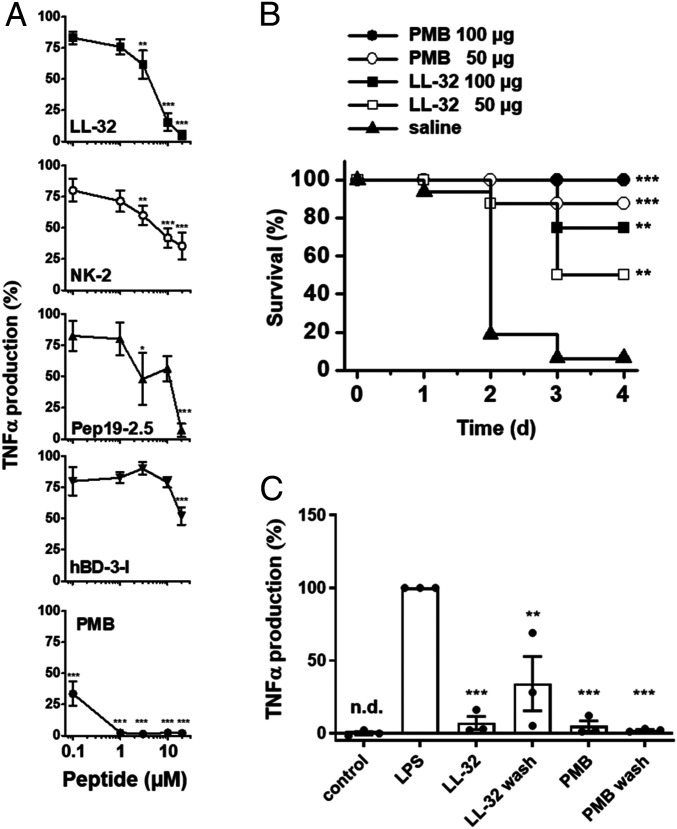
Fig. 1.
AMPs and PMB inhibit LPS-mediated inflammation in vitro and in vivo. (A) Human macrophages were incubated in medium alone or in the presence of 0.1, 1, 3, 10, and 20 µM concentrations of the peptides LL-32, NK-2, Pep19-2.5, hBD-3-l, or PMB for 30 min at 37 °C. The macrophages were subsequently stimulated with 5 nM LPS for 4 h at 37 °C. The concentrations of TNF-α were determined in supernatants. TNF-α values of samples stimulated with LPS in the absence of peptide were set 100% and all other values were calculated accordingly. The data are shown as the means ± SEM of n = 8 (LL-32), n = 4 to 5 (NK-2, Pep19-2.5, hBD-3-l), or n = 5 to 9 (PMB) independent experiments using cells from different donors. (B) Galactosamine-sensitized mice were injected intraperitoneally with LPS (100 ng/mouse; equivalent to 5 µg/kg) and subsequently with 50 or 100 µg/mouse of LL-32 or PMB or saline (n = 8 mice per group) at a different injection site. The survival of the mice was monitored daily. (C) Macrophages were incubated with 10 µM LL-32 or PMB for 30 min at 37 °C and subsequently washed three times with serum-free Roswell Park Memorial Institute (RPMI) 1640 medium to remove unbound peptide or left untreated, followed by stimulation with 5 nM LPS for 4 h. The concentrations of TNF-α in the supernatants were determined. TNF-α values of samples stimulated with LPS in the absence of peptide were set 100%, and all other values were calculated accordingly. The data are shown as the means ± SEM of n = 3 independent experiments using cells from different donors. Black dots represent the individual data points. Control, unstimulated cells; n.d., not detectable. Statistical analyses were performed via one-way ANOVA and Dunnett’s post test; *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 (peptide groups versus LPS control).