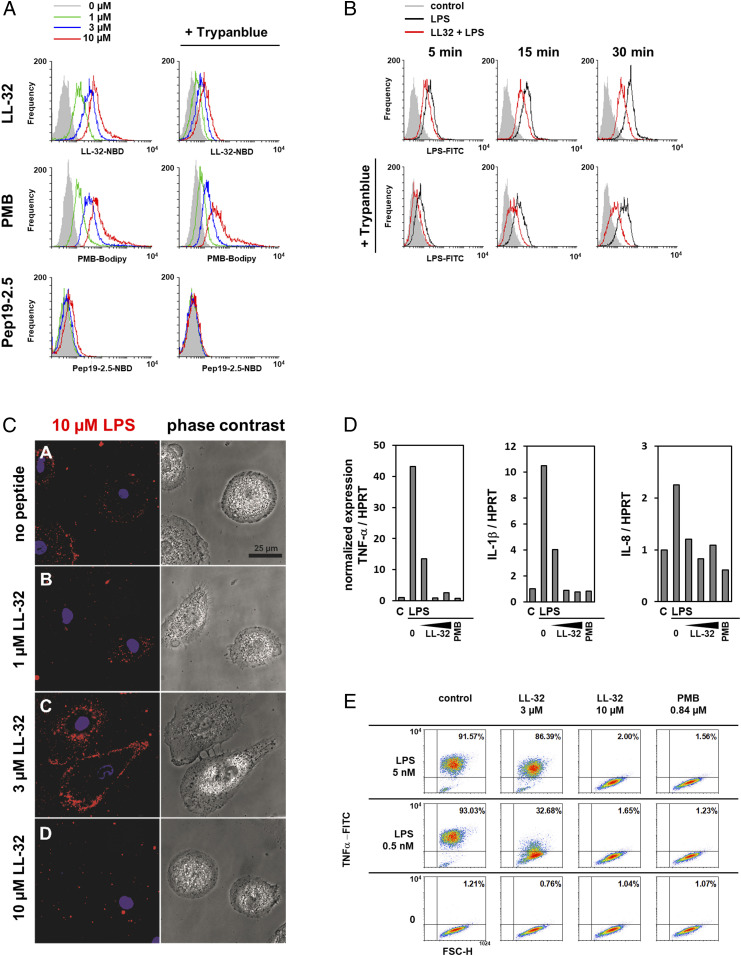
Fig. 2.
LL-32 and PMB binding modifies cellular LPS processing and inhibits inflammatory responses in human macrophages. (A) Macrophages were incubated with LL-32-NBD, Pep19-2.5-NBD, or PMB-BODIPY for 5 min at 37 °C, then washed and fixed. Subsequently, the samples were split and analyzed by flow cytometry to determine the total bound peptide directly and after quenching with 0.2% trypan blue to determine the amount of intracellular peptide. (B) Macrophages were incubated with 3 µM LPS-FITC in the presence of 3 µM LL-32 for 5, 15, or 30 min at 37 °C. The samples were analyzed by flow cytometry. (C) Macrophages cultured on µ-slides were stimulated with rhodamine-labeled LPS in the presence of 1, 3, and 10 µM LL-32 for 5 min at room temperature. Cell nuclei were counterstained with Hoechst. (Scale bar, 25 µM). (D) Macrophages were preincubated for 30 min with LL-32 (1, 3, and 10 µM) or PMB (1 µg/mL, 0.84 µM) and stimulated with 5 nM LPS for 1 h at 37 °C. Gene expression was analyzed by qRT-PCR. Data are relative expression ratios of the target to reference gene (HPRT), normalized to the untreated control. (E) Macrophages were preincubated with LL-32 (3 and 10 µM), PMB (1 µg/mL, 0.84 µM), or buffer (control) for 30 min at 37 °C and subsequently stimulated with LPS for 4 h in the presence of 10 µg/mL bafilomycin. Intracellular TNF-α was stained with a specific antibody, and the cells were analyzed by flow cytometry. Numbers in the upper right quadrants of plots indicate the percentages of gated macrophages positive for TNF-α. Data are representative of n = 3 (A, C–E) and n = 5 (B) independent experiments.