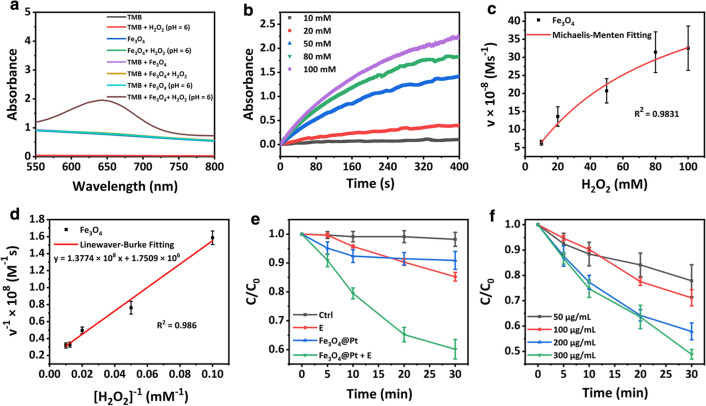
Fig. 3.
a UV–vis absorption spectra of the catalyzed oxidation of TMB (oxTMB) catalyzed by Fe3O4 NPs with 100 mM H2O2 under the reaction buffer (pH = 6). b Time-dependent absorbance changes at 652 nm as a result of the catalyzed oxidation of TMB at different H2O2 concentrations (10, 20, 50, 80, 100 × 10−3 M). c Michaelis–Menten kinetic analysis and d Lineweaver–Burk plotting for Fe3O4 with H2O2 as substrate. The steady-state catalytic rate (v) was calculated from the initial slopes of absorbance versus time plots in panel b. e Degradation rates of MB under different conditions ([Fe3O4@Pt]: 200 µg/mL for Fe3O4, AC output current: 10 mA, [MB]: 2.5 × 10−5 M). f Degradation rates of MB in the presence of Fe3O4@Pt NPs with different concentrations under electric field