Fig. (1). Workflow for developing small molecules targeting “hot segments” of PPIs in the central and peripheral nervous systems.
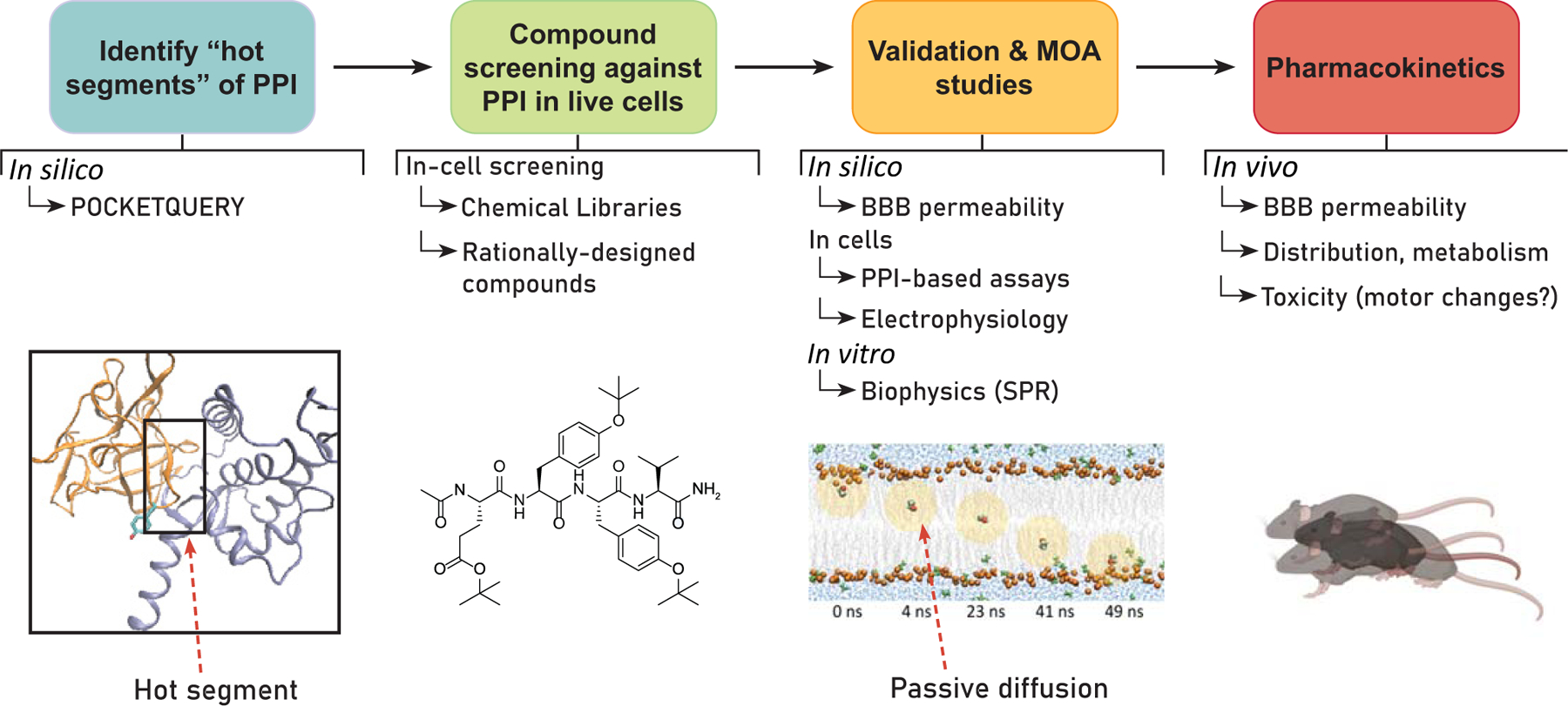
Phase 1) Based on existing structural data, potential “hot segments” of a PPI interface can be identified using in silico prediction methods, such as PocketQuery [24]. Phase 2) Screening of compounds against PPI interfaces with predicted “hot segments” using in-cell HTS platforms. Phase 3) After preliminary screening, hits are selected for orthogonal validation and mechanism of action (MOA) studies. These investigations can include in silico prediction of blood-brain barrier (BBB) permeability [26], in-cell assays to characterize PPI selectivity, electrophysiology to assess functional activity, and protein: ligand binding assays to determine binding affinities. Phase 4) Lead compounds identified are selected for in vivo pharmacokinetic (PK) and behavioral studies. Some in vivo studies of interest for the development of these modulators include single-unit electrophysiological recordings, assessments of mechanical hypersensitivity, and operant behaviors for the development of anti-epileptics, local anesthetics, and neuropsychopharmacological agents, respectively. Of particular interest for central nervous system (CNS) drug discovery, BBB permeability and deleterious effects on locomotor activity are assessed.
