Fig. (2). Diagrammatic representation of how “hot segments” of PPI interfaces between the CTD of Nav channels and intracellular fibroblast growth factors (iFGFs) function as allosteric sites for Nav channel modulation.
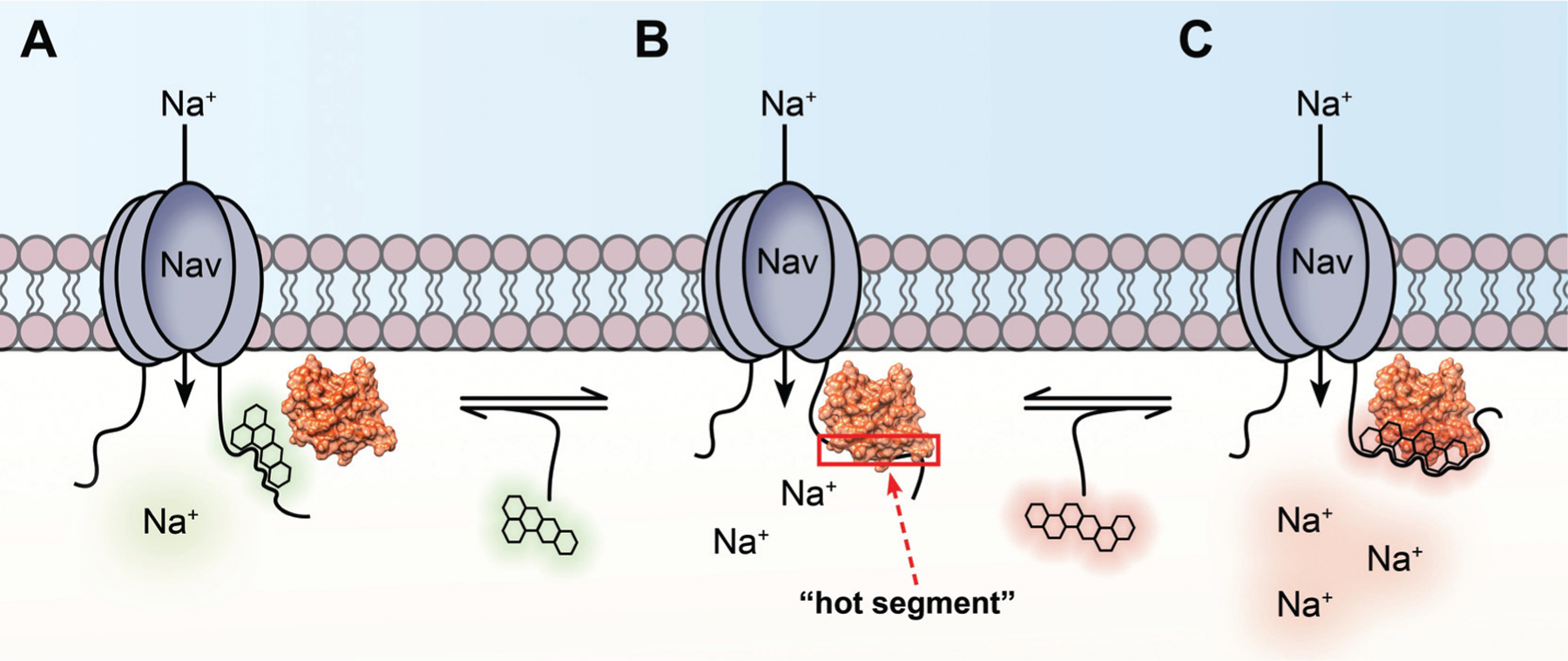
A) Small molecule targeting the iFGF:Nav PPI interface that inhibits association of the complex. Given that such PPIs are necessary for full physiological activity of Nav channels, such inhibition of complex assembly is anticipated to decrease Nav channel activity, resulting in molecules that inhibit the association of the complex functioning as negative allosteric modulators (NAM). B) Representation of a “hot segment” (red box) along an iFGF:Nav PPI interface that could serve as a druggable pocket for novel allosteric modulators of Nav channels. CTDs of Nav channel isoforms show less sequence homology relative to other structural components of the pore-forming α subunit. This amino acid sequence divergence among CTDs of Nav channel isoforms gives rise to structurally divergent PPI interfaces with auxiliary proteins, intermolecular interactions with functionally selective effects on different Nav channel isoforms. C) Small molecule targeting the iFGF:Nav PPI interface that stabilizes and inhibits dissociation of the complex. Inhibiting dissociation of the complex is anticipated to exacerbate iFGF-mediated regulatory effects on the channel, thereby leading to increased Nav channel activity. As such, molecules that inhibit dissociation of the complex are anticipated to function as positive allosteric modulators (PAM) of Nav channels.
