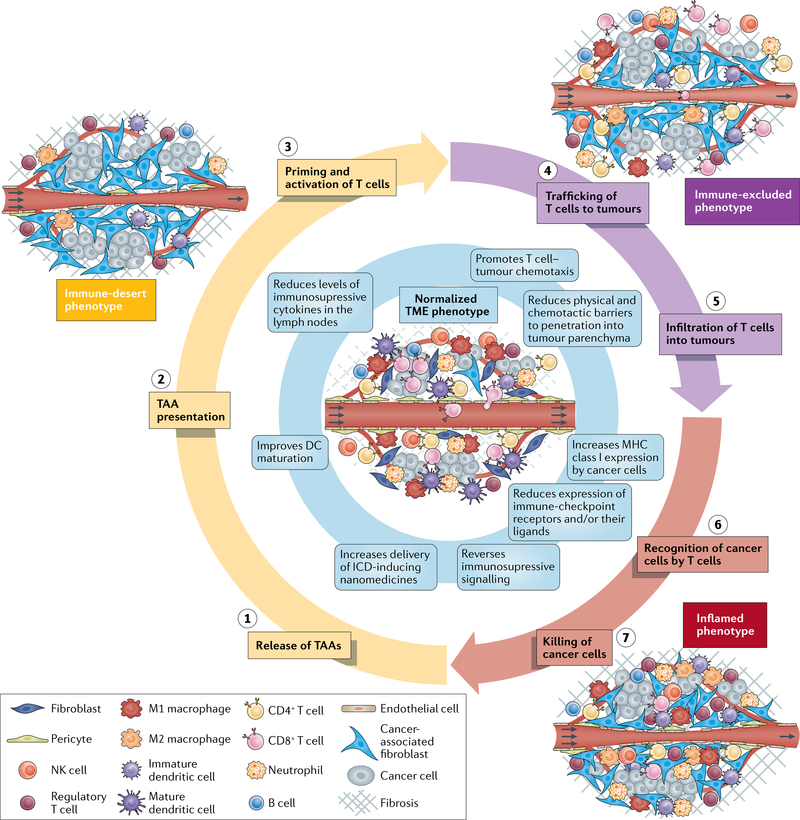
Fig. 1 |. Cancer-immunity TME phenotypes affecting responsiveness to immunotherapy18.
Three distinct cancer-immunity phenotypes exist and can affect responsiveness to immunotherapies in different ways. These phenotypes reflect tumours at different phases of the seven-step cancer-immunity cycle that must be completed repeatedly for immunotherapies to be effective. Tumour microenvironment (TME) normalization (centre, blue shading) promotes perpetuation of the cycle. In tumours of the immune-desert phenotype (yellow shading), the TME and the often limited number of immune cells within the tumour are immunosuppressed. The host immune system permits cancer cell growth owing to a lack of antigen recognition, immune tolerance and/or a failure to prime cytotoxic T cells. Some of these processes are affected by hypoxia. In tumours of the immune-excluded phenotype (purple shading), immune cell infiltration is restricted to the periphery and/or the stroma. The stromal factors that promote this phenotype similarly inhibit the delivery of nanomedicines and/or oxygen. In tumours of the inflamed phenotype (red shading), immune cells are stimulated by pro-inflammatory cytokines and are able to move throughout the tumour parenchyma. However, various inhibitory factors, which are often induced by hypoxia, lead to a reduction in antitumour immunity. Normalization of the vasculature by targeting angiogenic factors (such as VEGF and/or angiopoietin-2) and/or immune checkpoints and normalization of the tumour extravascular compartment by reprogramming cancer-associated fibroblasts (CAFs) to produce a less dense extracellular matrix (ECM) are two strategies that can be applied alone or in combination to normalize the entire TME and improve perfusion, oxygen delivery and drug distribution. Improper use of these strategies, however, can lead to excessive vessel pruning or CAF and/or ECM depletion, which might accelerate tumour progression and metastasis. Anti-programmed cell death 1 (PD-1) or programmed cell death 1 ligand 1 (PD-L1) antibodies can also normalize blood vessels in some tumour types and could promote the growth of mature blood vessels that are protected from pruning by antiangiogenic agents, which primarily target immature vessels. Signalling pathways such as those activated by VEGF, angiopoietin-2, CXCL12/CXCR4 and transforming growth factor-β, which can all be targeted to normalize the TME, are themselves immunosuppressive. As a result of these effects, the combination of therapies targeting these pathways with immunotherapies is a promising approach. Thus, specific TME normalization strategies based on the TME immune phenotype of the target tumour could increase both the response rates and the magnitude of responses to immunotherapies. DC, dendritic cell; ICD, immunogenic cell death; NK, natural killer; TAA, tumour-associated antigen.