Fig. (5). Angiotensin-(1–7) signaling attenuates protein O-GlcNAcylation in the retina.
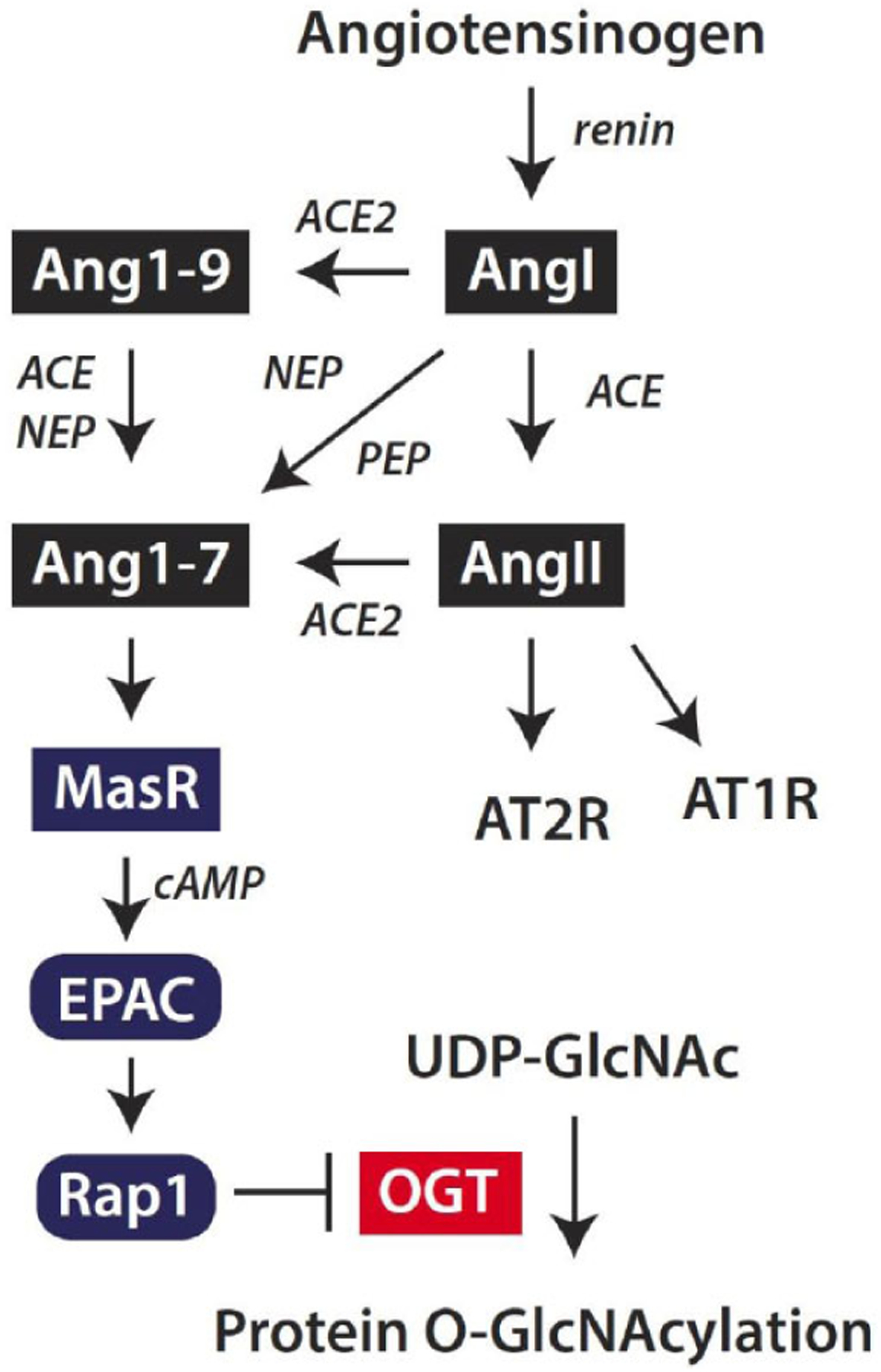
The classic renin-angiotensin system is initiated by cleavage of the pro-peptide angiotensinogen by renin to form Angiotensin I (AngI). AngI may be cleaved by angiotensin-converting enzyme (ACE) to generate angiotensin II (AngII), which acts via AT1 and AT2 receptors (AT1R and AT2R, respectively). Alternatively, Ang II may be cleaved by ACE2 to generate Angiotensin-(1–7) (Ang1–7). ACE2 also cleaves AngI to generate Ang1–9, which may be degraded by ACE or neutral endopeptidase (NEP) to produce Ang1–7. Finally, AngI may be degraded by endopeptidases such as NEP or prolyl endopeptidase (PEP) to directly form Ang1–7. Ang1–7 acts via the Mas receptor (MasR) to enhance cyclic AMP (cAMP) levels and suppress protein O-GlcNAcylation in retinal cells. Ang1–7 acts to suppress OGT activity via a signaling axis that includes the exchange protein activated by cAMP (EPAC) and Rap1.
