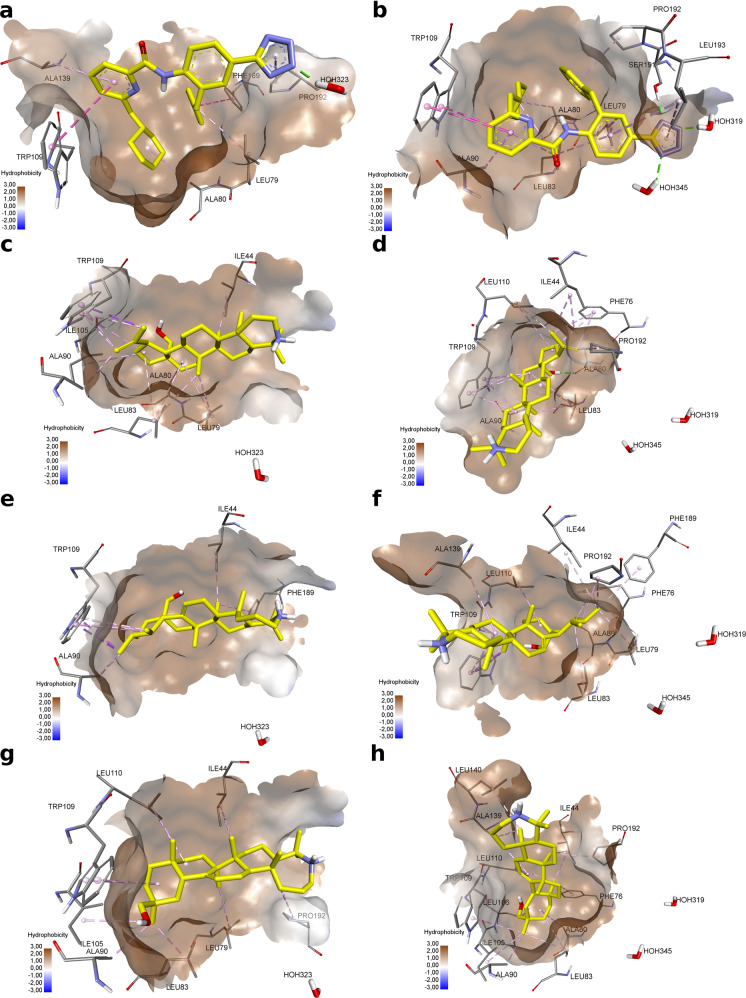
Fig. 3.
Docking of compounds 1, 8 and 15 in KSHV protease asymmetric monomers binding sites (monomer A (a, c, e, g); monomer B (b, d, f, h). a (−4.004 kcal/mol), b (−8.137 kcal/mol)—dimer disruptor; c, d compound 1; e, f compound 8; g, h compound 15. Noncovalent interactions of molecules are shown by dotted lines: green—hydrogen bonds, purple—stacking and pi-sigma interactions, pink—hydrophobic interactions