Figure 1. Three-dimensional structure and catalytic cycle of SmTGR.
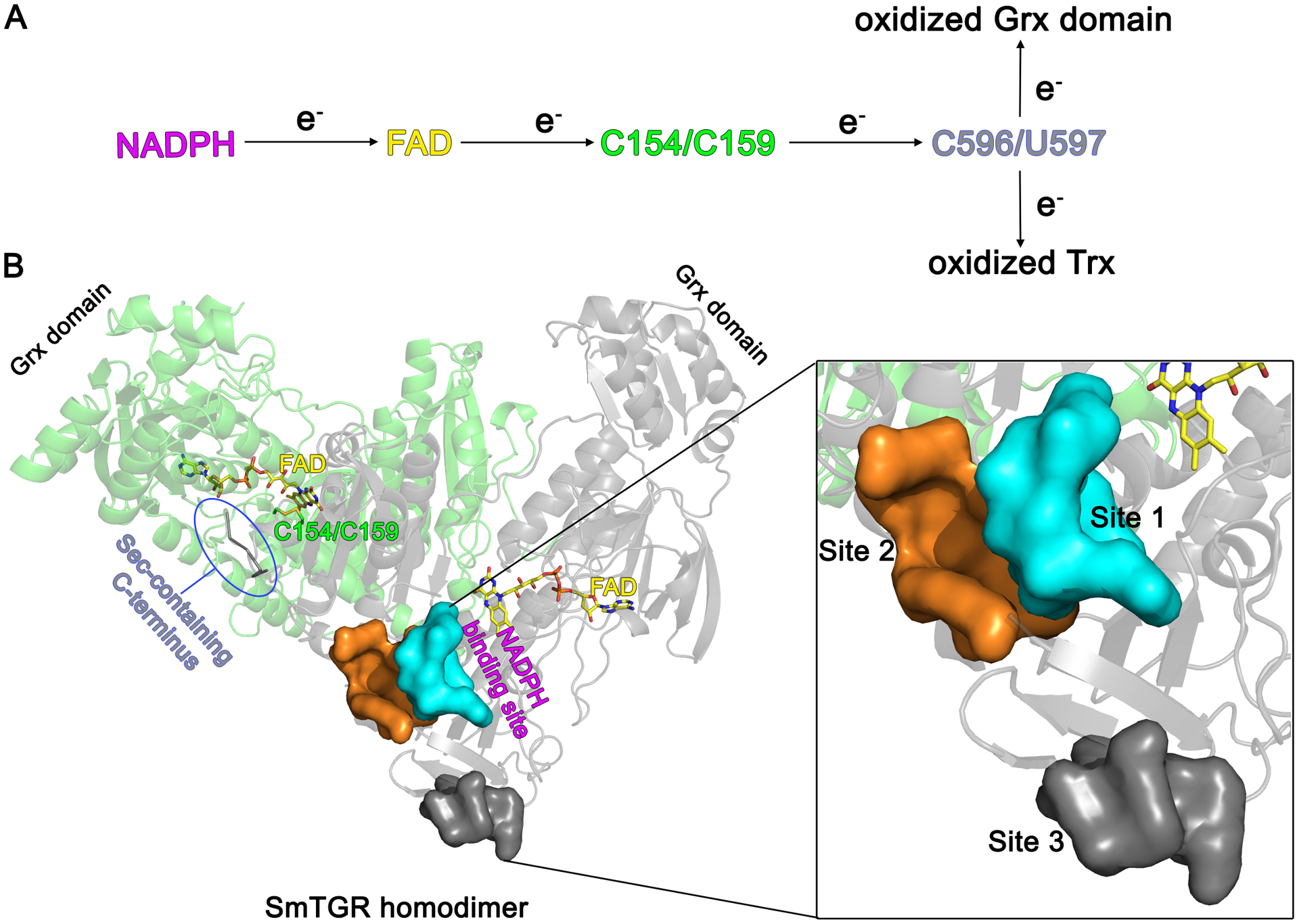
A. The electron flow within the active sites of SmTGR starting from NADPH and ending either with oxidized Trx or oxidized Grx domain is depicted. B. The subunits of the SmTGR homodimer are shown in green and grey cartoons together with the position of the main redox centers of the enzyme. The NADPH binding site extends from the re-face of the FAD, while C154/C159 and the C-terminus are found at the si-face of the co-factor. Localization of the three secondary sites described in this work with respect to the position of the FAD cofactor and of NADPH binding site is displayed in one subunit. The three secondary sites are shown by their solvent exposed surfaces in the magnification on the right.
