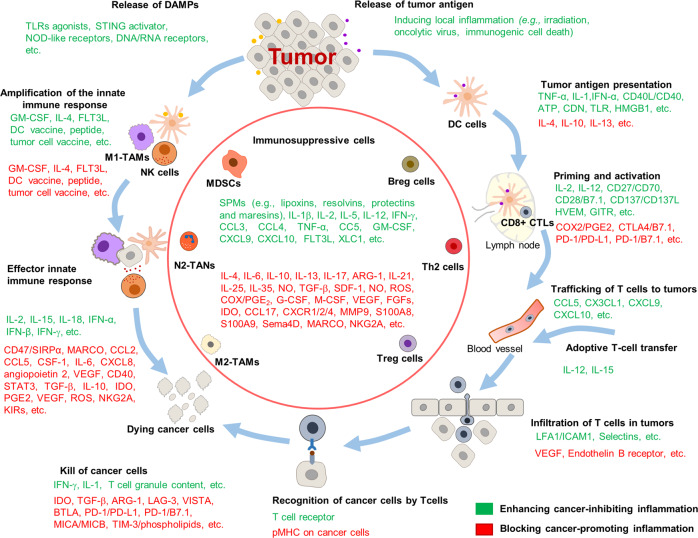
Fig. 4.
Harnessing the inflammation in cancer therapy. Several promising strategies for regulation of cancer-related inflammation have been suggested to improve anti-tumor response. On the one hand, inducing inflammation and modulating immune cell activation would overcome T-cell exclusion, turning “cold” tumors into “hot” tumors, for instance, local inflammation induced by irradiation or oncolytic viruses can promote innate immunity by activating nucleic-acid-sensing cytosolic receptors (cGAS–STING or RLRs) and subsequent type I IFN response. Besides, promoting DC maturation by cGAS–STING, TLR agonist, DC vaccine, or injection of GM-CSF can induce an acute inflammatory response and priming of T lymphocytes, which facilitate tumor regression. On the other hand, inhibiting chronic inflammation by anti-inflammatory drugs (e.g., aspirin) or accelerating inflammation resolution by proresolving mediators (SPM, e.g., lipoxins, resolvins, protectins, and maresins) also display an overall survival benefit for anti-cancer therapy. Furthermore, limitations of the infiltration and function of immunosuppressive cells (e.g., MDSCs, Treg cells, Breg cells, and M2-TAMs) by blocking inflammatory pathways is another way to restore immune surveillance and promote anti-tumor immunity. Green represents factors that enhancing these cancer-inhibiting inflammation, while red represents factors that blocking these cancer-promoting inflammation, would be beneficial to improve the effect of anti-tumor therapy