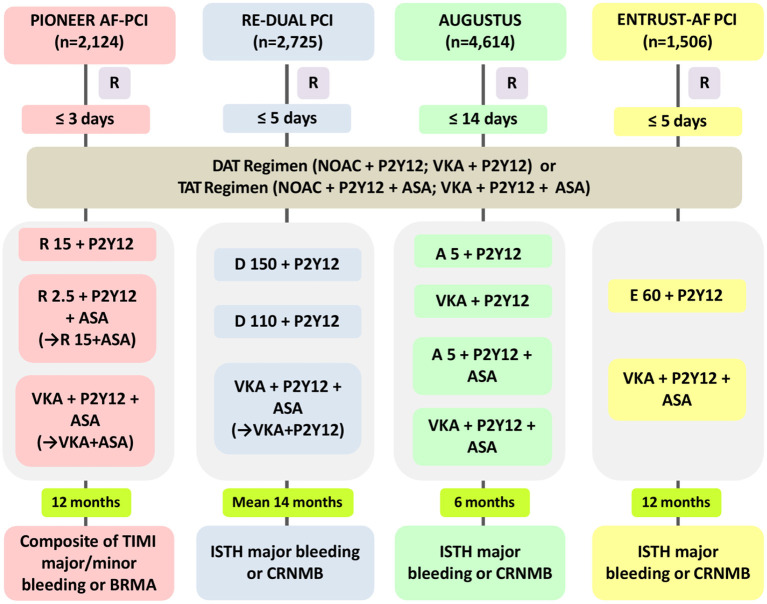
Figure 1.
Rationale and design of the four NOAC-based trials in AF patients with ACS or undergoing PCI. NOAC, non-vitamin K antagonist oral anticoagulant; PIONEER AF-PCI, Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention; RE-DUAL PCI, Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with Non-valvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; AUGUSTUS, Open-label, two-by-two Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in patients with Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention; ENTRUST-AF PCI, Edoxaban Treatment vs. Vitamin K Antagonist in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; R, time to randomization; DAT, dual antithrombotic therapy; TAT, triple antithrombotic therapy; R15, rivaroxaban 15 mg od; P2Y12, P2Y12 receptor inhibitor; R2.5, rivaroxaban 2.5 mg bid; ASA, aspirin; VKA, vitamin K antagonist; D150, dabigatran 150 mg bid; D110, dabigatran 110 mg bid; A5, apixaban 5 mg bid; E60, edoxaban 60 mg od; TIMI, thrombolysis in myocardial infarction; BRMA, bleeding requiring medical attention; CRNMB, clinically relevant non-major bleeding; ISTH, International Society on Thrombosis and Haemostasis.