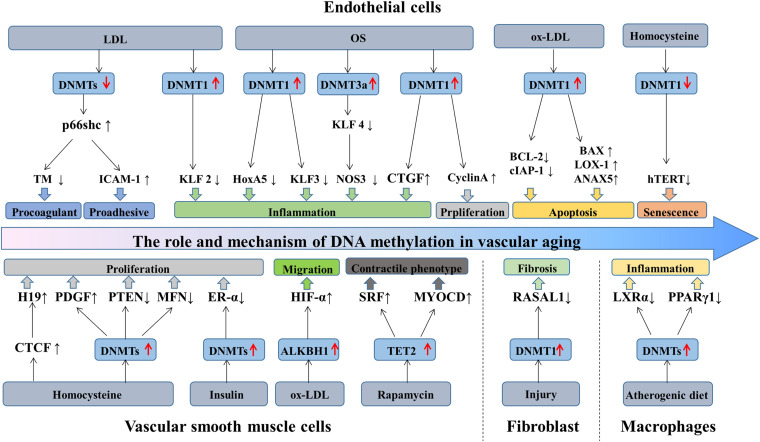
FIGURE 2.
The role and mechanism of DNA methylation in vascular aging. During aging, the structure and function of vascular cells change, causing ECs dysfunction, VSMCs proliferation and migration, fibroblasts differentiation, and macrophages inflammation. LDL stimulates p66shc expression by downregulating the activity of DNMTs and mediating the expression of ICAM-1 and TM, eventually exacerbating ECs dysfunction. Besides, LDL inhibits KLF2 expression via increasing DNMT1 activity, causing ECs inflammation. OS-treated ECs promotes DNMT1 activity and inhibits the generation of HoxA5 and KLF3. OS regulates KLF4, CTGF, and cyclinA methylation patterns by promoting DNMTs activity, causing ECs inflammation and proliferation. Ox-LDL triggers ECs apoptosis through the hypermethylation of cIAP-1 and BCL2 and the hypomethylation of BAX, LOX-1, and ANXA5. Homocysteine promotes ECs senescence by reducing hTERT expression via DNA methylation. Additionally, homocysteine increases CTGF and PDGF expression and decreases PTEN and MFN expression, mediating VSMCs proliferation. Insulin promotes VSMCs proliferation via ER-α hypermethylation. Ox-LDL promotes VSMCs migration by increasing HIF-1α generation through ALKBH1. TET2 overexpression promotes VSMCs differentiation by the upregulation of SRF and MYOCD expression. Besides, in response to injury, fibroblast activation through DNMT1 upregulation and RASAL1 hypermethylation. Furthermore, the hypermethylation of LXRα and PPARγ1 induces macrophages inflammation. DNMTs, DNA methyltransferases; TET, 10—11 translocations; LDL, low-density lipoprotein; OS, oscillatory shear stress; ECs, endothelial cells; VSMCs, vascular smooth muscle cells.