Abstract
We investigated the prognostic significance and treatment outcomes of pretreatment inflammatory response markers for locally advanced squamous cell carcinoma (SCC) of the external auditory canal (EAC) and middle ear (ME). Between July 2003 and July 2019, 21 patients with SCC of the EAC (n = 18) or ME (n = 3) who received radiotherapy with or without surgery or systemic therapy (radiotherapy alone [n = 2], radiotherapy + systemic therapy [n = 6], radiotherapy + surgery [n = 7], radiotherapy + surgery + systemic therapy [n = 6]) were retrospectively examined. The median radiation dose was 66.0 (range, 50.4–70.0) Gy, with daily fractions of 1.8–2.0 Gy. The median follow-up period was 25 months (range, 6–137). The two-year overall survival (OS), progression-free survival (PFS), and locoregional control (LC) rates were 61%, 48%, and 55%, respectively. OS, PFS, and LC did not differ significantly according to patient- (age, sex), tumor- (Pittsburgh stage, pretreatment neurological findings), and treatment-related (surgery or systemic therapy, radiation dose, prophylactic neck irradiation) factors. Conversely, there were significant differences in OS, PFS, and LC between patients with high and low pretreatment C-reactive protein-to-albumin ratios (p = 0.002, 0.003, and 0.004, respectively). OS also differed significantly between patients with high and low pretreatment neutrophil-to-lymphocyte ratios (NLR; p = 0.037). Other inflammatory response markers, including platelet-to-lymphocyte ratio (PLR) and albumin-to-globulin ratio (AGR), did not influence OS, PFS, or LC. Our findings suggest that pretreatment C-reactive protein-to-albumin ratio and NLRs have a significant impact on treatment outcomes in patients with locally advanced SCC of the EAC and ME.
Keywords: squamous cell carcinoma (SCC), external auditory canal (EAC), middle ear (ME), radiotherapy, inflammatory response marker, C-reactive protein-to-albumin ratio
INTRODUCTION
Squamous cell carcinoma (SCC) of the external auditory canal (EAC) and middle ear (ME) is a rare disease, with a reported prevalence of approximately one per million individuals [1, 2]. Although several treatment options have been proposed (surgery with or without postoperative chemoradiotherapy and definitive radiotherapy with or without chemotherapy), the optimal therapeutic strategy has not yet been established. Complete resection is usually recommended for SCC of the EAC and ME [3, 4].
Studies [5, 6] have reported an association between the host inflammatory response and cancer growth. Cancer progression requires interactions between cancer cells and their microenvironment. Systemic inflammatory response is associated with tumor microenvironment. It promotes microvascular regression and differentiation of cancer cells and suppresses the activity of host immune cells [7–11]. Thus, systemic inflammatory response may support tumor progression. Previous studies [12–14] have examined the prognostic significance of inflammatory response markers, including neutrophil-to-lymphocyte ratio (NLR), C-reactive protein (CRP)-to-albumin ratio (CAR), platelet-to-lymphocyte ratio (PLR), and albumin-to-globulin ratio (AGR), for various cancers. Recently, Li et al. [15] showed that preoperative NLR was significantly correlated with tumor recurrence in patients with SCC of the EAC. The authors concluded that preoperative NLR may be an unfavorable prognostic factor for SCC of the EAC. However, they analyzed the early Pittsburgh stage [16] without evaluating treatment outcomes according to the Pittsburgh stage. In this study, we aimed to evaluate the prognostic significance of pretreatment inflammatory response markers in patients with locally advanced SCC of the EAC or ME who received definitive or adjuvant radiotherapy.
MATERIALS AND METHODS
Ethical approval
The study design was approved by the appropriate ethical committee (approval number: 2010001), and all participants provided informed consent.
Patients
Between July 2003 and July 2019, 24 patients with locally advanced SCC of the EAC or ME who received radiotherapy with or without surgery or systemic therapy as initial treatment were retrospectively examined. Pretreatment NLR, CAR, PLR, and AGR data were available for 21 patients (male [n = 8], female [n = 13]) who were included in the analysis. The median age was 65 (range, 41–83) years. Eighteen patients had SCC of the EAC, and three patients had SSC of the ME. One patient had neck node metastases. None of the patients had distant metastases at presentation.
Pretreatment laboratory tests were usually performed within 10 (median, six [range, 1–18]) days before the start of initial treatment. All patients were diagnosed and staged based on findings of physical examination, computed tomography, and/or magnetic resonance imaging at presentation. These modalities were used in follow-up visits after the initial treatment to detect locoregional recurrence or distant metastases. Locoregional recurrence was defined as tumor regrowth in local and neck nodes. Progression-free survival (PFS) was defined as the time after treatment without deterioration of symptoms and disease, local regrowth, or appearance of metastatic lesions within or outside the irradiated field.
Treatments
Treatment strategies were determined based on the Pittsburgh staging system [16] and patients’ general condition. Seven patients received radiotherapy and surgery; six received radiotherapy, surgery, and systemic therapy; six received radiotherapy and systemic therapy; and two received radiotherapy alone. The median radiation doses for the definitive radiotherapy with or without systemic therapy regimen and radiotherapy and surgery with or without systemic therapy regimen were 66.0 (range, 66.0–70.0) and 66.0 (range, 50.4–70.0) Gy, respectively. Twelve patients received local irradiation and nine received local and prophylactic neck irradiation. In principle, radiotherapy and systemic therapy consisted of cisplatin (80 mg/m2 on day one every three weeks). Ten patients received fluoropyrimidine-based chemotherapy (tegafur/gimeracil/oteracil/potassium [n = 3], tegafur/uracil [n = 2], and 5-fluorouracil [n = 5]) with or without cisplatin. In addition, two patients received cetuximab after radiotherapy.
Statistical analyses
Survival rates were calculated from the start of initial treatment using the Kaplan–Meier method. The log-rank test was used to evaluate differences in overall survival (OS), PFS, and locoregional control (LC). Pretreatment NLR, CAR, PLR, and AGR values were calculated. There are no established cutoff values for NLR, CAR, PLR, and AGR in SCC of the EAC and ME. To determine the optimal cutoff values for predicting OS, PFS, and LC in patients with SCC of the EAC and ME, receiver operating characteristic (ROC) curve analysis was performed. The areas under the ROC curves for OS and PFS were 0.62 (sensitivity, 90%; specificity, 45%), 0.70 (sensitivity, 55%; specificity, 65%), 0.55 (sensitivity, 70%; specificity, 55%), and 0.62 (sensitivity, 60%; specificity, 73%) for NLR, CAR, PLR, and AGR, respectively. The areas under the ROC curves for LC were 0.54 (sensitivity, 100%; specificity, 25%), 0.59 (sensitivity, 44%; specificity, 98%), 0.53 (sensitivity, 56%; specificity, 67%), and 0.56 (sensitivity, 89%; specificity, 33%) for NLR, CAR, PLR, and AGR, respectively. For OS and PFS, an NLR of 3.95, CAR of 0.31, PLR of 216, and AGR of 1.34 corresponded to the maximum sum of sensitivity and specificity. For LC, an NLR of 4.43, CAR of 0.31, PLR of 135.9, and AGR of 1.40 corresponded to the maximum sum of sensitivity and specificity. Statistical analyses were conducted using JMP software, version 14.3.0 (SAS Institute, Cary, NC, USA).
RESULTS
Patients’ characteristics are presented in Table 1. The median follow-up period was 25 (range, 6–137) months. The one- and two-year OS (Fig. 1), PFS (Fig. 2), and LC rates were 81% and 61%, 52%, 48%, 68%, and 55%, respectively. Nine patients experienced tumor recurrence. All nine patients had in-field recurrences. Five patients had in-field recurrence alone, three had in-field recurrence and regional lymph node metastasis outside the radiation field, and one had in-field recurrence and distant metastasis. One patient with neck node metastases who was treated with concurrent chemoradiotherapy had no recurrence or distant metastasis at the last follow-up.
Table 1.
Patient characteristics
| Characteristic | Value | |
|---|---|---|
| No. of patients | 21 | |
| Age | median 65 (41–83) | |
| < 65 years | 10 (47.6%) | |
| ≥ 65 years | 11 (52.4%) | |
| Aex | male | 8 (38.1%) |
| female | 13 (61.9%) | |
| PS (ECOG) | 0 | 17 (80.9%) |
| 1 | 3 (14.3%) | |
| 3 | 1 (4.8%) | |
| Pittsburgh staging | T3 | 10 (47.6%) |
| T4 | 11 (52.4%) | |
| Neurological findings before treatment | yes | 8 (38.1%) |
| no | 13 (61.9%) | |
| Surgery | yes | 13 (61.9%) |
| subtotal resection | 10 (47.6%) | |
| complete resection | 3 (14.3%) | |
| no | 8 (38.1%) | |
| Systemic therapy | yes | 10 (47.6%) |
| no | 11 (52.4%) | |
| Radiation dose (Gy) | median 66 (50.4–70.0) | |
| < 66 | 4 (19.0%) | |
| ≥ 66 | 17 (81.0%) | |
| Irradiated field | local | 12 (57.1%) |
| local + prophylactic | 9 (42.9%) | |
| NLR | median 2.33 (1.02–12.9) | |
| < 3.95 | 16 (76.2%) | |
| ≥ 3.95 | 5 (23.8%) | |
| CAR | median 0.04 (0.002–0.84) | |
| < 0.31 | 16 (71.4%) | |
| ≥ 0.31 | 5 (28.6%) | |
| PLR | median 153.1 (41.8–504.9) | |
| < 216 | 12 (57.1%) | |
| ≥ 216 | 9 (42.9%) | |
| AGR | median 1.32 (0.88–2.0) | |
| < 1.34 | 11 (52.4%) | |
| ≥ 1.34 | 10 (47.6%) |
EAC; external auditory canal, ME; middle ear, PS (ECOG); performance status (Eastern Cooperative Oncology Group), NLR; neutrophil-to-lymphocyte ratio, CAR; C-reactive protein-to-albumin ratio, PLR; platelet-to-lymphocyte ratio, AGR; albumin-to-globulin ratio.
Values are number (percentage) or median (range).
Fig. 1.
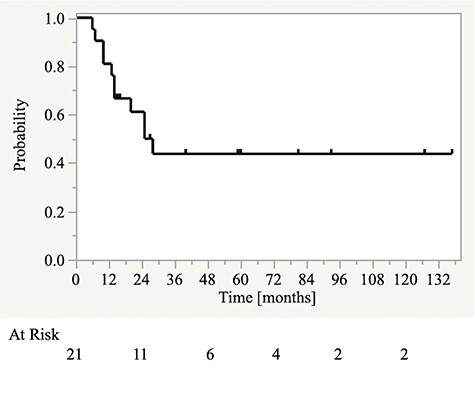
Kaplan–Meier curves for overall survival.
Fig. 2.
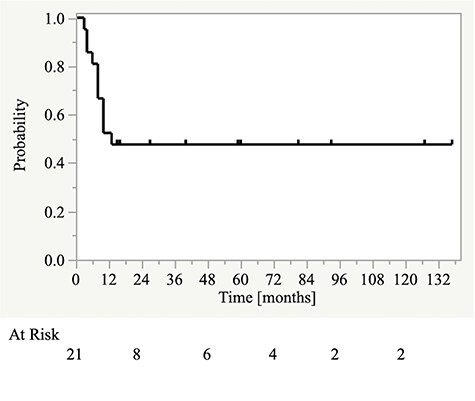
Kaplan–Meier curves for progression-free survival.
Survival and LC rates stratified by Pretreatment NLR
The one-year OS rate was significantly different between patients with NLR <3.95 and those with NLR ≥3.95 (94% vs 40%; p = 0.037). Conversely, the one-year PFS rate was not significantly different between the two groups (63% vs 20%; p = 0.160) (Fig. 3a and Table 2). There was also no significant difference in the one-year LC rate between patients with NLR <4.43 and those with NLR ≥4.43 (69% vs 75%; p = 0.902) (Table 3).
Fig. 3.
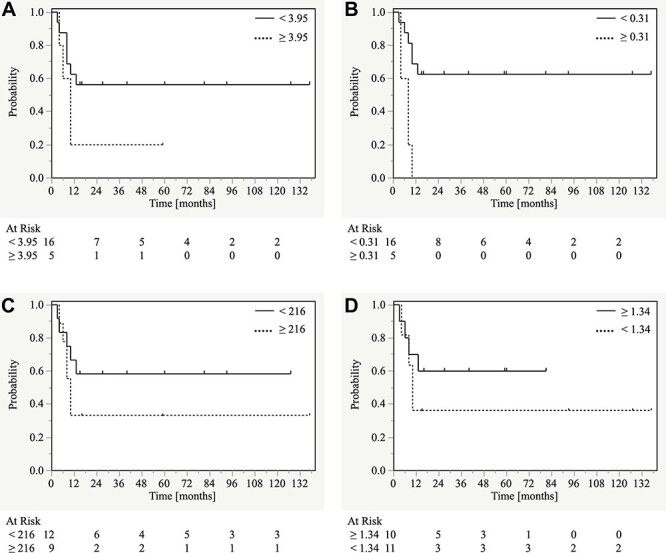
Kaplan–Meier curves for progression-free survival according to pretreatment inflammatory response markers. (A) Neutrophil-to-lymphocyte ratio (<3.95 vs ≥3.95), (B) C-reactive protein-to-albumin ratio (<0.31 vs ≥0.31), (C) platelet-to-lymphocyte ratio (<216 vs ≥216), and (D) albumin-to-globulin ratio (<1.34 vs ≥1.34)
Table 2.
Univariate analysis of overall survival and progression-free survival rates
| Variables | OS | DFS | |||||
|---|---|---|---|---|---|---|---|
| One-year (%) | Two-year (%) | p | One-year (%) | Two-year (%) | p | ||
| Age | < 65 years (n = 10) | 80 | 58 | 0.9737 | 50 | 50 | 0.7912 |
| ≥ 65 years (n = 11) | 82 | 64 | 55 | 45 | |||
| Sex | male (n = 8) | 75 | 63 | 0.7274 | 50 | 38 | 0.4961 |
| female (n = 13) | 85 | 59 | 54 | 54 | |||
| Pittsburgh staging | T3 (n = 10) | 80 | 70 | 0.4986 | 70 | 60 | 0.2486 |
| T4 (n = 11) | 82 | 55 | 36 | 36 | |||
| Neurological findings before treatment | yes (n = 8) | 88 | 63 | 0.8889 | 50 | 50 | 0.8762 |
| no (n = 13) | 77 | 61 | 54 | 46 | |||
| Surgery | yes (n = 13) | 85 | 61 | 0.9332 | 46 | 46 | 0.8117 |
| no (n = 8) | 75 | 63 | 63 | 50 | |||
| Systemic therapy | yes (n = 9) | 78 | 65 | 0.8968 | 44 | 44 | 0.8652 |
| no (n = 12) | 83 | 58 | 58 | 50 | |||
| Radiation dose (Gy) | < 66 Gy (n = 4) | 75 | - | 0.0747 | 25 | - | 0.3079 |
| ≥ 66 Gy (n = 17) | 82 | 70 | 59 | 53 | |||
| Irradiated field | local (n = 12) | 83 | 58 | 0.1593 | 42 | 33 | 0.1209 |
| local + prophylactic (n = 9) | 78 | 67 | 67 | 67 | |||
| NLR | < 3.95 (n = 16) | 94 | 74 | 0.0365 | 63 | 56 | 0.1603 |
| ≥ 3.95 (n = 5) | 40 | 20 | 20 | 20 | |||
| CAR | < 0.31 (n = 16) | 88 | 74 | 0.0019 | 69 | 63 | 0.0025 |
| ≥ 0.31 (n = 5) | 60 | 20 | 0 | 0 | |||
| PLR | < 216 (n = 12) | 92 | 74 | 0.129 | 67 | 58 | 0.2853 |
| ≥ 216 (n = 9) | 67 | 44 | 33 | 33 | |||
| AGR | < 1.34 (n = 11) | 73 | 44 | 0.1993 | 36 | 36 | 0.3666 |
| ≥ 1.34 (n = 10) | 90 | 80 | 70 | 60 | |||
OS; overall survival, PFS; progression-free survival, NLR; neutrophil-to-lymphocyte ratio, CAR; C-reactive protein-to-albumin ratio, PLR; platelet-to-lymphocyte ratio, AGR; albumin-to-globulin ratio.
Table 3.
Univariate analysis of locoregional control rates
| Variables | LC | |||
|---|---|---|---|---|
| One-year (%) | Two-year (%) | p | ||
| Age | < 65 years (n = 10) | 79 | 53 | 0.5625 |
| ≥ 65 years (n = 11) | 58 | 58 | ||
| Sex | male (n = 8) | 75 | 60 | 0.9379 |
| female (n = 13) | 64 | 51 | ||
| Pittsburgh staging | T3 (n = 10) | 79 | 79 | 0.1862 |
| T4 (n = 11) | 57 | 34 | ||
| Neurological findings before treatment | yes (n = 8) | 58 | 58 | 0.6727 |
| no (n = 13) | 75 | 54 | ||
| Surgery | yes (n = 13) | 65 | 43 | 0.7230 |
| no (n = 8) | 75 | 75 | ||
| Systemic therapy | yes (n = 9) | 65 | 65 | 0.7374 |
| no (n = 12) | 73 | 36 | ||
| Radiation dose (Gy) | < 66 Gy (n = 4) | 38 | - | 0.3707 |
| ≥ 66 Gy (n = 17) | 74 | 60 | ||
| Irradiated field | local (n = 12) | 52 | 35 | 0.0560 |
| local + prophylactic (n = 9) | 89 | 76 | ||
| NLR | < 4.43 (n = 17) | 69 | 53 | 0.9018 |
| ≥ 4.43 (n = 4) | 75 | 75 | ||
| CAR | < 0.31 (n = 16) | 87 | 69 | 0.0035 |
| ≥ 0.31 (n = 5) | 0 | 0 | ||
| PLR | < 135.9 (n = 8) | 88 | 58 | 0.8190 |
| ≥ 135.9 (n = 13) | 53 | 53 | ||
| AGR | < 1.40 (n = 13) | 59 | 39 | 0.0792 |
| ≥ 1.40 (n = 8) | 86 | 86 | ||
LC; locoregional control, NLR; neutrophil-to-lymphocyte ratio, CAR; C-reactive protein-to-albumin ratio, PLR; platelet-to-lymphocyte ratio, AGR; albumin-to-globulin ratio.
Survival and LC rates stratified by Pretreatment CAR
The one-year OS rate was significantly different between patients with CAR <0.31 and those with CAR ≥0.31 (88% vs 60%; p = 0.002). The one-year PFS rate was also significantly different between the two groups (69% vs 0%; p = 0.003) (Fig. 3b and Table 2). Furthermore, there was a significant difference in the one-year LC rate between patients with CAR <0.31 and those with CAR ≥0.31 (87% vs 0%; p = 0.004) (Table 3).
Survival and LC rates stratified by Pretreatment PLR
The one-year OS rate was not significantly different between patients with PLR <216 and those with PLR ≥216 (92% vs 67%; p = 0.129). The one-year PFS rate was also not significantly different between the two groups (67% vs 33%; p = 0.285) (Fig. 3c and Table 2). Furthermore, there was no significant difference in the one-year LC rate between patients with PLR <135.9 and those with PLR ≥135.9 (88% vs 53%; p = 0.819) (Table 3).
Survival and LC rates stratified by pretreatment AGR
The one-year OS rate was not significantly different between patients with AGR <1.34 and those with AGR ≥1.34 (73% vs 90%; p = 0.199). The one-year PFS rate was also not significantly different between the two groups (36% vs 70%; p = 0.367) (Fig. 3d and Table 2). Furthermore, there was no significant difference in the one-year LC rate between patients with AGR <1.40 and those with AGR ≥1.40 (59% vs 86%; p = 0.079) (Table 3).
Survival and LC rates according to patient-, tumor-, and treatment-related factors
OS and PFS did not differ significantly according to age (<65 vs ≥65 years), sex, Pittsburgh stage (T3 vs T4), radiation dose (<66.0 vs ≥66.0 Gy), irradiated fields (with or without prophylactic neck irradiation), use of surgery, and addition of systemic therapy (Table 2). Furthermore, there were no significant differences in LC according to age, sex, Pittsburgh stage, radiation dose, irradiated fields, use of surgery, and addition of systemic therapy (p = 0.563, 0.938, 0.186, 0.371, 0.056, 0.723, and 0.737, respectively) (Table 3).
DISCUSSION
In this study, the two-year OS and PFS rates for patients with locally advanced SCC of the EAC and ME were approximately 60% and 50%, respectively. The OS and PFS curves plateaued by two and one years from the start of treatment, respectively. There were no significant differences in OS or PFS rates according to patient-, tumor-, and treatment-related factors. However, there were significant differences in both OS and PFS between the high and low CAR groups. Similarly, there was a significant difference in OS between the high and low NLR groups.
Chronic inflammation in the tumor microenvironment can promote malignant tumor progression [17]. In this study, pretreatment CAR was significantly associated with OS and PFS in patients with locally advanced SCC of the EAC and ME. A previous meta-analysis [4] showed that a high CAR is associated with a relatively poor outcome in patients with solid tumors, including those with head and neck tumors. However, the relationship between CAR and treatment outcomes in patients with locally advanced SCC of the EAC and ME has not been well-documented. Serum CRP and albumin levels can be measured in peripheral blood samples. CRP is produced by hepatocytes as a systemic response to cytokines, particularly interleukin-6. It is released from leukocytes within the tumor microenvironment and has been associated with progressive disease and relatively poor survival in patients with different types of cancer [18–20]. In addition, inflammation is associated with decreased serum albumin levels owing to suppressed liver function, resulting in reduced albumin production. The release of cytokines from inflammatory cells may increase microvascular permeability and increase the flow of serum albumin into the extravascular compartment [21]. These findings suggest that CAR is useful for assessing the extent of inflammation in the tumor microenvironment and predicting prognosis. The present findings indicate that CAR is associated with treatment outcomes in patients with locally advanced SCC of the EAC and ME. Overall, this evidence suggests that prognostication of locally advanced SCC of the EAC and ME requires the assessment of tumor- and treatment-related factors, as well as those related to the tumor microenvironment.
In this study, there was no significant difference in PFS between the high and low pretreatment NLR, PLR, and AGR groups. However, a low NLR and PLR and a high AGR were associated with improved PFS. A previous meta-analysis [22] showed that a high NLR was associated with a poor outcome in patients with SCC of the head and neck. In this study, significant differences in OS, but not PFS, were observed between patients with a high and low NLR. Therefore, NLR may be relevant for the prognostication of locally advanced SCC of the EAC and ME. Previous studies [23, 24] have shown that pretreatment PLR and AGR affect prognosis of several types of cancer. However, the role of these markers in the present context remains unclear and requires further validation.
A previous study [25] showed that definitive chemoradiotherapy may achieve comparable outcomes to surgical resection with or without radiotherapy. In this study, no significant differences in outcomes were observed between patients who did and did not undergo surgery. When complete resection is difficult, definitive chemoradiotherapy may improve outcomes of patients with locally advanced SCC of the EAC and ME. However, in this study, the chemotherapy and combination therapy regimens were heterogeneous and the sample size was small; thus, further studies are required to determine the effectiveness of chemoradiotherapy.
This study had some limitations associated with its retrospective nature. First, the sample size was small. Although previous studies have reported significant associations between several prognostic factors and outcomes of patients with SCC of the EAC and ME [3, 16, 26, 27], this study did not replicate these findings, likely because of the small sample size. However, an association between pretreatment CAR and outcomes was observed, despite the small sample size. Consequently, we believe that pretreatment CAR may be useful for prognostication. Nevertheless, further studies are required to validate this association in locally advanced SCC of the EAC and ME. Second, this study included patients undergoing different types of treatment. As there are no standard treatments for locally advanced SCC of the EAC and ME, the study population was necessarily heterogeneous. Surgery was performed wherever possible; definitive chemoradiotherapy was added as the preferred treatment modality for patients in whom complete resection was difficult to achieve.
In conclusion, in this study, pretreatment CAR was associated with OS and PFS in patients with locally advanced SCC of the EAC and ME. Pretreatment NLR was also associated with OS. No relationship was observed between tumor- or treatment-related factors and OS or PFS.
Contributor Information
Kenji Makita, Department of Radiology, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan; Departments of Radiation Oncology, National Hospital Organization Shikoku Cancer Center, Kou-160, Minami-Umenomoto-Machi, Matsuyama, Ehime 791-0280, Japan.
Yasushi Hamamoto, Departments of Radiation Oncology, National Hospital Organization Shikoku Cancer Center, Kou-160, Minami-Umenomoto-Machi, Matsuyama, Ehime 791-0280, Japan.
Noriko Takata, Department of Radiology, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
Hirofumi Ishikawa, Department of Radiology, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
Shintaro Tsuruoka, Department of Radiology, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
Kotaro Uwatsu, Department of Radiology, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
Naohito Hato, Department of Otorhinolaryngology, Head and Neck Surgery, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
Teruhito Kido, Department of Radiology, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1. Acharya PP, Sarma D, McKinnon B. Trends of temporal bone cancer: SEER database. Am J Otolaryngol 2020;41:102297. [DOI] [PubMed] [Google Scholar]
- 2. Brant JA, Eliades SJ, Chen J et al. Carcinoma of the middle ear: a review of the national cancer database. Otol Neurotol 2017;38:1153–7. [DOI] [PubMed] [Google Scholar]
- 3. Pfreundner L, Schwager K, Willner J et al. Carcinoma of the external auditory canal and middle ear. Int J Radiat Oncol Biol Phys 1999;44:777–88. [DOI] [PubMed] [Google Scholar]
- 4. Wu J, Tan W, Chen L et al. Clinicopathologic and prognostic significance of C-reactive protein/albumin ratio in patients with solid tumors: an updated systemic review and meta-analysis. Oncotarget 2018;9:13934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 8. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 9. Kim S, Miller BJ, Stefanek ME et al. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: relevance to cancer-related fatigue. Cancer 2015;121:2129–36. [DOI] [PubMed] [Google Scholar]
- 10. Baniyash M. Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer Immunol Immunother 2016;65:857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Raaf PJ, Sleijfer S, Lamers CH et al. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: an explorative study. Cancer 2012;118:6005–11. [DOI] [PubMed] [Google Scholar]
- 12. Saito H, Kono Y, Murakami Y et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin and neutrophil–lymphocyte ratio in gastric cancer patients. World J Surg 2018;42:1819–25. [DOI] [PubMed] [Google Scholar]
- 13. Tsujino T, Komura K, Hashimoto T et al. C-reactive protein-albumin ratio as a prognostic factor in renal cell carcinoma – A data from multi-institutional study in Japan. Urol Oncol 2019;37:812.e1–8. [DOI] [PubMed] [Google Scholar]
- 14. Azab B, Kedia S, Shah N et al. The value of the pretreatment albumin/globulin ratio in predicting the long-term survival in colorectal cancer. Int J Colorectal Dis 2013;28:1629–36. [DOI] [PubMed] [Google Scholar]
- 15. Li F, Shi X, Dai C. Prognostic value of pre-operative peripheral inflammation markers in patients with squamous cell carcinoma of the external auditory canal. Braz J Otorhinolaryngol 2020;S1808-8694:30088–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch BE. Staging system revision. Arch Otolaryngol Head Neck Surg 2002;128:93–4. [PubMed] [Google Scholar]
- 17. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–35. [DOI] [PubMed] [Google Scholar]
- 18. Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg 2001;182:197–201. [DOI] [PubMed] [Google Scholar]
- 19. Hefler LA, Concin N, Hofstetter G et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res 2008;14:710–4. [DOI] [PubMed] [Google Scholar]
- 20. Nagaoka S, Yoshida T, Akiyoshi J et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int 2007;27:1091–7. [DOI] [PubMed] [Google Scholar]
- 21. Fanali G, di Masi A, Trezza V et al. Human serum albumin: from bench to bedside. Mol Aspects Med 2012;33:209–90. [DOI] [PubMed] [Google Scholar]
- 22. Takenaka Y, Oya R, Kitamiura T et al. Prognostic role of neutrophil-to-lymphocyte ratio in head and neck cancer: a meta-analysis. Head Neck 2018;40:647–55. [DOI] [PubMed] [Google Scholar]
- 23. Zhou X, Du Y, Huang Z et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014;9:e101119, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He J, Pan H, Liang W et al. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: a systematic review and meta-analysis. J Cancer 2017;8:4002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takenaka Y, Cho H, Nakahara S et al. Chemoradiation therapy for squamous cell carcinoma of the external auditory canal: a meta-analysis. Head Neck 2015;37:1073–80. [DOI] [PubMed] [Google Scholar]
- 26. Ogawa K, Nakamura K, Hatano K et al. Treatment and prognosis of squamous cell carcinoma of the external auditory canal and middle ear: a multi-institutional retrospective review of 87 patients. Int J Radiat Oncol Biol Phys 2007;68:1326–34. [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa T, Kumamoto Y, Natori Y et al. Squamous cell carcinoma of the external auditory canal and middle ear: an operation combined with preoperative chemoradiotherapy and a free surgical margin. Otol Neurotol 2006;27:242–8 discussion 249. [DOI] [PubMed] [Google Scholar]


