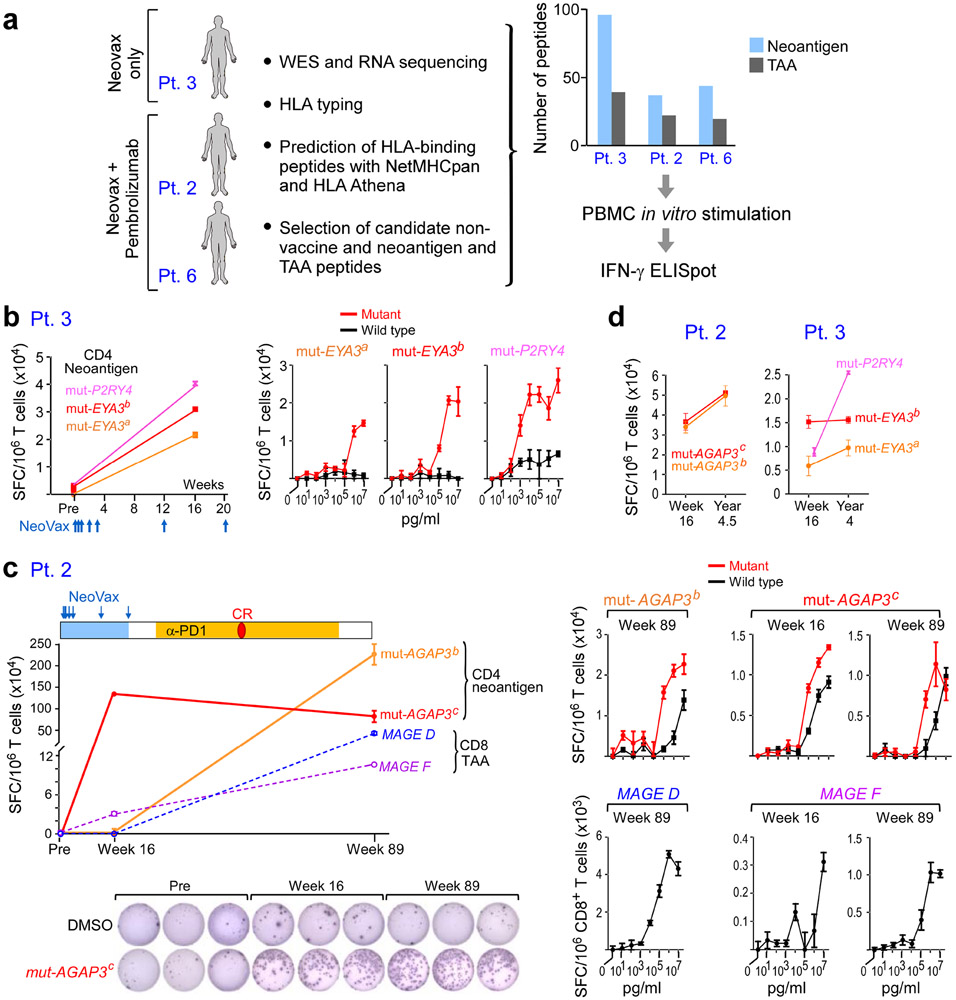
Figure 6. Vaccine-induced T cell responses spread to non-vaccine neoantigen and TAA epitopes.
a. Somatic mutations and tumor-associated antigens were identified by WES of melanoma and germline DNA and their expression was confirmed by tumor RNA-seq. Non-vaccine neoantigen and tumor-associated antigen (TAA) epitope spreading peptides were selected on the basis of HLA binding predictions (Methods). PBMCs were pre-stimulated in vitro with epitope spreading peptides and reactivity was confirmed by IFN-γ ELISpot. b. Left: Pt. 3 IFN-γ secretion of CD4+ T cells stimulated with 3 non-vaccine neoantigen peptides measured by ELISpot in triplicates pre-vaccination and at week 16. Right: IFN-γ secretion of neoantigen-specific CD4+ T cells tested across a range of concentrations of mutated and wildtype peptides. c. Top left: Pt. 2 IFN-γ secretion of T cells stimulated with 2 non-vaccine neoantigen peptides (solid lines) and 2 TAA peptides (dashed lines) as measured by ELISpot in triplicates at week 16 and week 89 (after anti-PD-1 therapy). CR indicates complete response. Bottom left: Representative IFN-γ ELISpot response of mut-AGAP3c-specific CD4+ T cells. Right: IFN-γ secretion of neoantigen-specific CD4+ T cells tested across a range of concentrations of mutated and wildtype peptides; TAA-specific CD8+ T cells against TAA peptides (lower panels), respectively. d. IFN-γ ELISpot responses of CD4+ T cells specific for non-vaccine neoantigens persist up to 3 years post-vaccination in Pts. 2 and 3. ELISpots were performed in triplicate wells/condition (error bars, s.e.m).