Scheme 2. Synthesis Route for Different Trisubstituted Isoxazoles (C-5 Library).
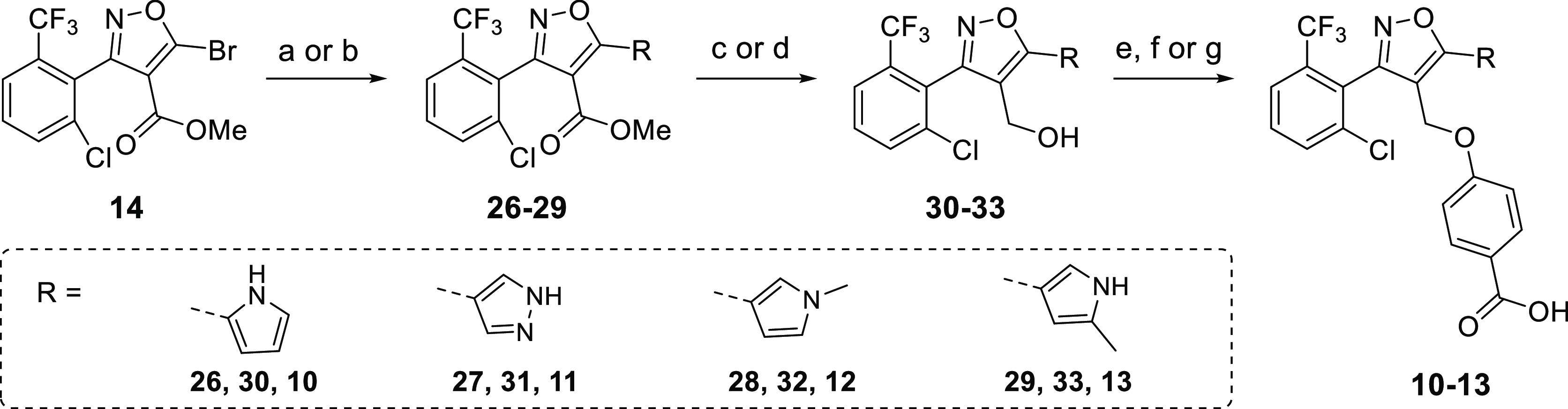
Reagents and conditions: (a) N-Boc-pyrrole-B(pin), N-THP-pyrazole-B(pin) or 5-methyl N-Boc-pyrrole-B(pin) (see Experimental Section for the synthetic procedure of this pinacol ester), Pd(dppf)Cl2, Cs2CO3, and DME, 85 °C, 8 h, 49% (26), 34% (27), and 24% (29); (b) N-methyl-pyrrole-B(pin), Pd(PPh3)4, Na2CO3, DME, and H2O, 85 °C, 8 h, 55% (28); (c) DIBAL and CH2Cl2, −78 °C, 3 h, 69% (30), 71% (31), and 58% (33); (d) LiAlH4 and THF, 0 °C → rt, 2 h, 76% (32); (e) (i) methyl 4-hydroxybenzoate, DIAD, PPh3, and THF, reflux, 3 h, 21%, (ii) LiOH, EtOH, and H2O, 95 °C, 3 h, 41% (10); (f) (i) methyl 4-hydroxybenzoate, DIAD, PPh3, Et3N, and THF, reflux, 3 h, 44–45%, (ii) LiOH, EtOH, and H2O, 95 °C, 3 h, 86% (12), 83% (13); (g) (i) methyl 4-hydroxybenzoate, DIAD, PPh3, Et3N, and THF, reflux, 3 h, 49%, (ii) TFA and CH2Cl2, 40 °C, 2 h, 87%, (iii) LiOH, EtOH, and H2O, 95 °C, 3 h, 89% (11).
