Abstract
Rising trends in the incidence and mortality of early-onset CRC in those who are ages less than 50 years have been well-established. These trends have spurred intense investigation focused on elucidating the epidemiology and characteristics of early-onset CRC, as well on identifying strategies for early detection and prevention. In this review, we provide a contemporary update on early-onset CRC with a particular focus on epidemiology, molecular characterization, red flag signs and symptoms, and screening for early-onset CRC.
INTRODUCTION
In the United States (US), >140,000 individuals will be diagnosed with colorectal cancer (CRC) this year, and as many as 1 in 7 of these will be under the age of 50, deemed early-onset CRC.1 Since the 1990s, early-onset CRC incidence has been rising at an alarming rate in the United States, and the cause of this rapid increase, which primarily impacts birth cohorts born after the1950s, remains largely unexplained.1 This has spurred intense investigation focused on early-onset CRC, as recently reviewed in Gastroenterology by Stoffel and Murphy.2 Given the complexity, wide range, and rapid pace of research addressing early-onset CRC, in this review we provide a contemporary update on early-onset CRC with a particular focus on the epidemiology, molecular characterization, red flag signs and symptoms, and screening for early-onset CRC (Table 1).
Table 1.
Summary of the current state of knowledge for the epidemiology, tumor marker characterization, red flag symptoms and screening for early-onset CRC
| What is known? | What is new? | What is unknown? | |
|---|---|---|---|
| Epidemiology |
|
|
|
| Tumor markers |
|
|
|
| Red flag symptoms |
|
|
|
| Screening |
|
|
|
EPIDEMIOLOGY OF EARLY-ONSET CRC
Trends in Early-Onset CRC Incidence and Mortality
It is well-established that early-onset CRC incidence has increased over the past three decades in the United States.1 CRC incidence rates have increased by nearly 45% in adults ages 20–49 years, from 8.6 per 100,000 in 1992 to 13.1 per 100,000 in 2016 in the United States.2 Equally concerning is that CRC mortality rates among adults younger than 50 years increased by 1.3% per year from 2008–2017, whereas CRC mortality rates declined by 3% per year in individuals aged 65 years and older; decreases in mortality have slowed to 0.6% per year in individuals ages 50 to 64 years.1 Although incidence patterns for early-onset CRC are similar in men and women over the past few decades, incidence varies by site (predominantly rectal and distal colon), stage (more late-stage disease), race, ethnicity, and geographic residence.1, 3–7
Since last year’s review in Gastroenterology,2 there is new information on the geographic variation of early-onset CRC incidence in the United States and worldwide. Overall, early-onset CRC incidence remains highest in southern and rural parts of the country.4, 5, 7 Much of the United States (40 of 47 states) reported increases in early-onset CRC among non-Hispanic Whites from 1995 to 2015 with the most rapid rise in western states.5 In contrast to non-Hispanic Whites, the incidence of early-onset CRC among Blacks and Hispanics was relatively stable between 1995–2015 across most states.5 New data suggest that globally, early-onset CRC is increasing in high-income countries including Australia, New Zealand, Canada, Korea, Taiwan, Germany, Denmark, Slovenia, Sweden, and the United Kingdom.8–11 Conversely, early-onset CRC incidence has declined in three high-income countries: Italy, Austria, and Lithuania. Intriguingly, Austria adopted an earlier CRC screening initiation age (starting at age 40 years) in 2003 utilizing fecal-based testing and colonoscopy for screening,12 raising the possibility that early initiation of screening in countries experiencing increasing incidence might be an effective strategy for addressing this problem.10
Early-Onset CRC Risk Factors
Several risk factors have been hypothesized as potential drivers of early-onset CRC based on global temporal trends in these risk factors, with the hypothesis that some of these risk factors exert effects through impacts on colonic inflammation and the gut microbiome. Specifically, lifestyle factors such as a Western diet,13, 14 alcohol,15 and tobacco16 are risk factors for early-onset CRC or advanced colorectal neoplasia. Recent work from the Nurses’ Health Study found prolonged sedentary television viewing, a surrogate for an inactive lifestyle, was associated with an increased risk of early-onset CRC, particularly for rectal cancer.17 Using the same cohort, obesity was associated with a nearly 2-fold higher risk of early-onset CRC (adjusted odds ratio (aOR): 1.93, 95% CI: 1.15–3.25).18 Novel work from Korea also suggests that adults diagnosed with diabetes at ages <50 years have a 27% increased risk of advanced neoplasia compared to those without diabetes (aOR: 1.27, 95% CI: 1.06–1.54); however, this study relied on a surrogate outcome of neoplasia given the limited number of patients with early-onset CRC (N=14) in their cohort.19 Overall, these results suggest that many of the established risk factors for late-onset CRC also play a role in early-onset CRC.
In contrast to these and other findings reported in last year’s review in Gastroenterology,2 two studies from an academic medical center and the Veteran Affairs population showed no association between obesity or diabetes and early-onset CRC.20, 21 Conflicting results can be partially explained by study design differences. Specifically, the two recent case-control studies ascertained body mass index information at or near the time of CRC diagnosis, which may have contributed to the lack of association due to reverse causality.22 Another recent study using the IBM MarketScan Commercial database found that metabolic syndrome was associated proximal early-onset colon cancer (aOR 1.37; 95% CI: 1.04–1.81), but not with early-onset distal colon or rectal cancers.23 Novel work from the Nurses’ Health Study II also suggests that a Western diet is associated with an increased risk of early-onset high-risk adenomas (aOR: 1.67; 95% CI: 1.18–2.37, highest vs lowest quintile), particularly in the distal colon and rectum.24 Other potential risk factors for early-onset CRC that have been proposed, but remain understudied, include antibiotic exposure, perceived stress, red and processed meats, synthetic food coloring, and food additives (e.g., monosodium glutamate, titanium dioxide, high-fructose corn syrup, emulsifiers, etc.).25, 26 Opportunities for advancing the knowledge base regarding risk factors include a need for more studies reporting risk associations stratified by CRC location (i.e., colon versus rectum), as well as studies exploring relationships between exposures that occur during development, from conception to early adulthood, and early-onset CRC.
SOMATIC MARKERS IN EARLY-ONSET CRC TUMORS
Somatic markers, in the form of molecular characteristics found within early-onset CRCs, can provide clues regarding potential etiologies and inform targeted treatment approaches. About 10–20% of early-onset CRC tumors are characterized as having high microsatellite instability (MSI-high),27–29 and these early-onset MSI-high tumors are predominantly attributed to germline mutations associated with Lynch Syndrome.27 Although hereditary CRC syndromes are associated with an increased risk of early-onset CRC,30 the majority of early-onset CRC is not attributable to germline mutations in cancer risk genes,29 and the molecular profile of these sporadic early-onset tumors is distinct from late-onset CRC. As detailed by Stoffel and Murphy,2 compared to late-onset CRC, early-onset CRC has a lower prevalence of somatic APC and BRAF mutations and a higher prevalence of somatic CTNNB1 mutations. Early-onset CRC tumors are also more likely to exhibit epigenetic changes indicative of global hypomethylation of DNA than late-onset CRC. Among the consensus molecular subtypes (CMS) for CRC, early-onset CRC has a high proportion of the CMS-1 subtype, which is characterized by tumors containing MSI-high and inflammatory or immunogenic markers.2
Recent studies support these findings and provide additional information on the heterogeneity of molecular markers among those with early-onset CRC, by age at diagnosis.31 They also provide evidence that the serrated pathway to CRC, which includes sessile serrated lesions, is not a major pathway driving early-onset CRC.31, 32 One of these studies was conducted among CRC patients who were referred to targeted next-generation tumor sequencing, including 4,668 CRC patients who were ages < 50 years and 13,550 who were ages ≥ 50 years.32 This large study sample allowed for stratification by MSI-status; among MSI-high tumors, BRAF was mutated in 48% of older patients and only 5% of younger patients. Given that the CRC serrated pathway involving sessile serrated lesion precursors is characterized by MSI-high, BRAF-mutated tumors,33 it is unlikely that sessile serrated lesions are prominent precursors for early-onset CRC. As such, an increase in incidence of sessile serrated lesions under age 50 is unlikely to explain the rising incidence of early-onset CRC.
Another recent study applied a “multiomics” approach to analyzing molecular markers for early-onset CRC by conducting in-depth analyses of 233 microsatellite stable tumors, including analyses in tissue, plasma, and serum.34 Results based on gene expression levels in tissue, protein plasma markers, and inflammatory markers in serum all suggest that oxidative stress mediated by deficiencies in NRF2 activity is likely an important pathway for early-onset CRC development. NRF2 plays a crucial role in preventing toxicity and the accumulation of reactive oxygen species which can result in oxidative stress and inflammation.35 Taken together with the aforementioned observed high frequency of CMS-1 subtype tumors, these findings add to the growing body of evidence that inflammatory pathways may be particularly important to the development of early-onset CRC. These observations may have clinical and research implications, as anti-inflammatory medications, like aspirin, should be further evaluated in the context of early-onset CRC prevention and treatment.36
In addition to providing insights as to the pathogenesis and potential etiologies of early-onset CRC, research on somatic markers may also guide therapy. For example, cartilage oligomeric matrix protein (COMP) expression in early-onset CRC promotes cellular proliferation and tumorigenesis and leads to the hypothesis that COMP may be a potential treatment target in early-onset CRC.37, 38 Use of tumor mutational burden as a somatic marker is another area of interest, because high tumor mutational burden is associated with a higher likelihood of response to immune checkpoint inhibitors.39 In CRC, MSI-high tumors and those that harbor somatic mutations in POLE are associated with higher tumor mutational burden.39 This link is of interest, because somatic POLE mutations are more common in early-onset CRC, and identifying POLE mutations and associated high tumor mutation burden may guide selection of therapies such as immune checkpoint inhibitors in the future.40
Despite important advances in the molecular characterization of early-onset CRC, the etiologic and treatment implications of many early-onset CRC somatic tumor markers remain unclear. Future work to expand mutational signatures analyses and link mutational signatures in early-onset CRC to etiologic processes may shed light on factors driving the increase in early-onset CRC.41 Research on somatic markers associated with early-onset CRC also has potential to inform oncologic therapies, which is particularly important given the high proportion of individuals with early-onset CRCs who present at an advanced stage.42
EARLY-ONSET CRC RED FLAG SIGNS AND SYMPTOMS
Red flag signs or symptoms precede 70–95% of early-onset CRC cases.43–46 Rectal bleeding is the most commonly reported red flag symptom in early-onset CRC cases, with abdominal pain, change in bowel habits (including constipation and diarrhea), unexplained weight loss, and anemia also frequently reported.43, 45, 47–49 Despite these common presentations, few studies have examined whether, on average, these red flag signs or symptoms are predictive of early-onset CRC. A recent study comparing early-onset CRC cases to later-onset CRC cases and controls found abdominal pain, rectal pain, change in bowel habits, rectal bleeding, and weight loss were associated with increased early-onset CRC risk.50 Another recent study found rectal bleeding and iron deficiency anemia associated with 10-fold increased early-onset CRC risk.51
While red flag signs and symptoms may confer increased risk for CRC, recognition and work up may be delayed. Recent evidence has shown an average 6-month time to diagnosis from symptom presentation in early-onset CRC patients.48, 52–54 There are several potential explanations for the increased time to diagnosis. One patient-level explanation is lack of risk awareness, where the patient believes that he or she is “too young” to worry about cancer.54 Additionally, a lack of access to primary care or health insurance is a potential barrier to timely work-up.54 One provider-specific explanation is dismissal of symptoms or misattribution of symptoms to more benign conditions, such as hemorrhoids when rectal bleeding is present.55, 56 Though conventional wisdom and best practice may suggest that diagnostic work-up should be performed with minimal delay, studies have not yet linked delays in diagnosis with worse early-onset CRC stage at presentation or five-year survival.53, 54
Nonetheless, symptomatic presentation tends to reflect advanced CRC stage at diagnosis and potentially worse prognosis, making standard diagnostic work-up strategies critical to rule out early-onset CRC. For example, the American Society for Gastrointestinal Endoscopy recommends flexible sigmoidoscopy for patients with rectal bleeding under age 40 and full colonoscopy for those ages 40 and older.57 For iron deficiency anemia, the American Gastroenterological Association recommends that men and postmenopausal women, and suggests that premenopausal women, receive diagnostic evaluation that includes colonoscopy.58 Additionally, we recommend that pending further data, individuals with otherwise unexplained weight loss or abdominal pain should have early-onset CRC considered as part of the differential diagnosis. Colonoscopy may not be the primary strategy for work up of weight loss or abdominal pain in many cases, but it should be considered if other work up (such as abdominal imaging) or interventions to address these symptoms do not result in a diagnosis and symptom resolution. To avoid potential for overwhelming colonoscopy capacity, care must be taken to carefully triage individuals towards colonoscopy vs. targeted or expectant management. While normally used as a screening tool, findings published last month from a National Health Service randomized trial also found the quantitative fecal immunochemical test (FIT), coupled with a low threshold for positivity, could be an effective triage method to rule out CRC in symptomatic individuals, particularly as a non-invasive test during the COVID-19 pandemic.59 Additional research is needed to determine the effectiveness of this approach for evaluation of individuals with red flag symptoms potentially suggestive of early-onset CRC.
Taken together, the large burden of symptomatic early-onset CRC and long time to diagnosis necessitates an actionable work-up plan. We suggest a framework for identifying and addressing potential red flag signs and symptoms of early-onset CRC in which: 1) Red flag signs and symptoms are systematically recognized as including early-onset CRC as part of the differential diagnosis; 2) Triage to immediate colonoscopy vs alternative diagnostic strategies or immediate sign/symptom-specific therapy based on clinical guidelines, severity of presentation, and other clinical factors; and 3) Timely, systematic follow up to confirm a diagnosis other than CRC or symptom resolution with referral to colonoscopy in cases that remain unresolved after 60 days for individuals initially triaged away from immediate colonoscopy (Figure 1). Successful implementation of this framework requires buy-in from providers and a multidisciplinary commitment to this approach which may include the use of outreach to patients and providing patients with convenient options for follow-up, including telehealth visits or secure messaging.
Figure 1. A framework for identifying and addressing “red flag” signs and symptoms of early-onset CRC.
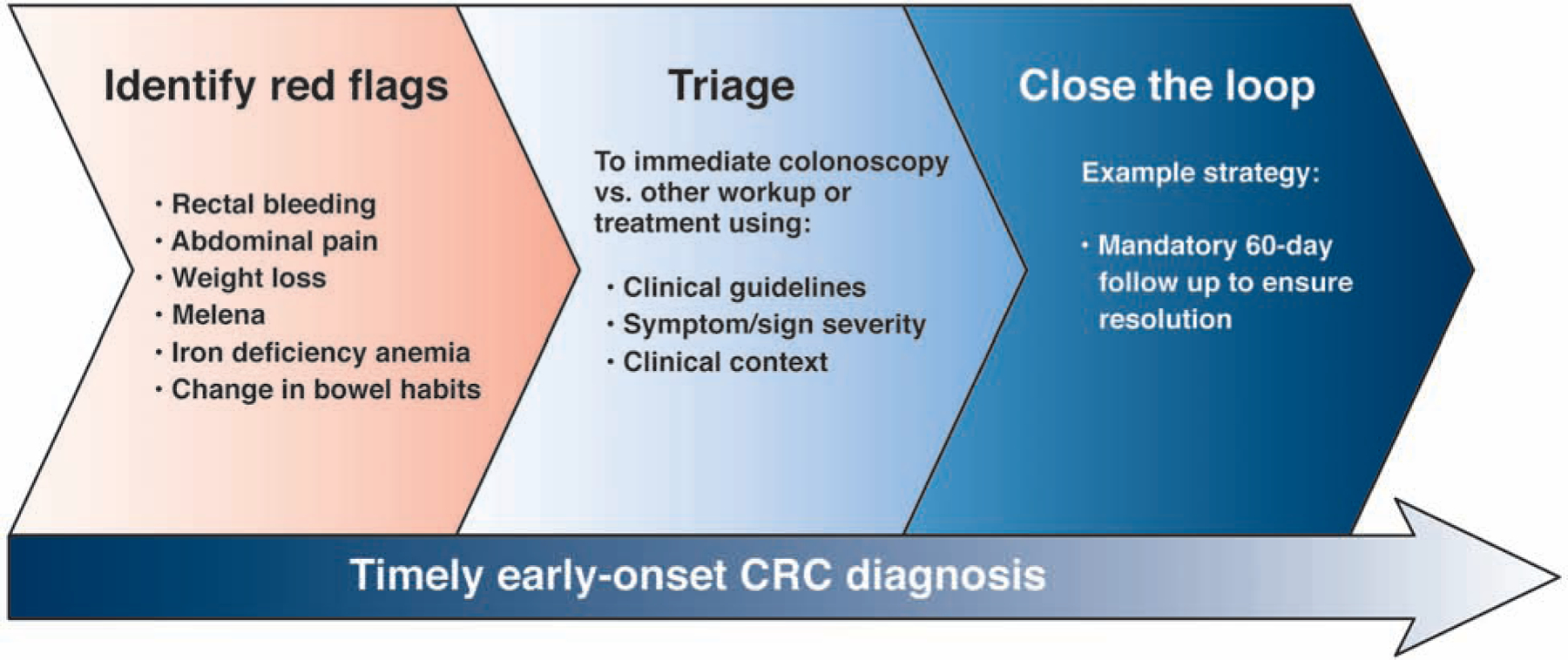
To ensure timely early-onset diagnosis, we propose to 1) increase awareness of potential red flags associated with early-onset CRC; 2) actively triage every patient with a red-flag to either immediate colonoscopy or other work up and treatment based on clinical guidelines, symptom/sign severity, and clinical context; and 3) Closing the clinical loop for all patients with red-flags not triaged immediately to colonoscopy with clinical follow up such as a mandatory 60 day clinic visit to ensure resolution of the red flag or referral to colonoscopy.
PRECISION AND POPULATION-BASED SCREENING FOR EARLY-ONSET CRC
Two strategies that may be applied for early detection and prevention of early-onset CRC among asymptomatic young adults are: 1) precision screening based on genetics, lifestyle, family history, and other factors, and 2) population-based screening for individuals otherwise at average risk. Early screening initiation based on family history has been the primary precision screening strategy recommended for early detection and prevention of early-onset CRC. The importance of family history-based recommendations is underscored by a recent study reporting that 1 in 4 early-onset CRC cases ages 40–49 met CRC family history criteria for early screening, and that 98% of those who met family history criteria could have had CRC diagnosed earlier (or possibly even prevented) if earlier screening had been implemented.60 The findings also highlight that 3 out of 4 early-onset CRC cases did not have a family history, suggesting additional precision screening strategies may be required to optimize early detection and prevention.
More sophisticated approaches which may in the future inform precision screening for early-onset CRC draw on more complex family history-based risk models, genetic risk scores, as well as diet, lifestyle, environment, and constitutional factors. Using nationwide Swedish family-cancer data, one study found that taking into account the exact number and age of presentation of affected relatives with CRC provides a more accurate estimate of at what age an unaffected relative might reach a substantial 10-year risk for incident cancer, and that these ages often varied substantially from the age of recommended screening initiation based on current guidelines.61 Genetic risk scores used for risk stratification are calculated based on the aggregate risk associated with having common genetic variants. Individually, variants present contribute only small increases in CRC risk, but when considered in aggregate, the burden of variants present can result in substantial increases in risk. Use of risk scores covering genetic, lifestyle, and other factors resulted in a wide range of recommended ages for screening initiation, from 41 to 71 years, for those without a family history at highest to lowest risk, respectively in a recent study.62 Among individuals without a family history, an increased genetic risk score could identify individuals with up to 4.3-fold increased risk compared to those with a low score in another recent study.63 A limitation of all of the aforementioned studies was a focus on populations having exclusively European ancestry. Further, whether more complex strategies for risk stratification can be practically implemented has not been demonstrated.64 Nonetheless, these results raise the exciting possibility that factors other than family history have promise for precision screening recommendations and other preventive interventions, particularly for those younger than age 45.
The new draft recommendation by the US Preventive Services Task Force to initiate screening at age 45 instead of 50,65 congruent with the American Cancer Society’s qualified recommendation for this approach,66 heralds a new era of opportunities and challenges for population screening. If US Preventive Services Task Force accepts this as a final Grade A or B recommendation, this would effectively require most insurers to cover screening beginning at age 45 without cost-sharing.26 These recommendations were mainly based on modeling studies accounting for rising early-onset CRC incidence and mortality which predict that earlier initiation will avert 1 additional CRC death per 1000 people beginning regular screening at age 45 instead of 50.67, 68 The recommendations are further bolstered by recent work suggesting that the yield for advanced neoplasia is similar for both for “average risk equivalent” 45 to 49 year-olds to 50 to 54 year-olds undergoing routine colonoscopy,69 as well as among average risk 45 to 49 year-old Blacks compared to average risk Blacks and non-Hispanic Whites age 50 to 54 undergoing colonoscopy for an abnormal FIT.70 Earlier initiation of screening has also recently been predicted to be cost effective71, and, if ultimately issued as a final recommendation by USPSTF, will offer a consistent recommendation for earlier initiation for Blacks and Alaska Natives, two groups which have an earlier uptick in age-specific CRC incidence72, 73 and higher CRC mortality.1
To realize the full promise of this new opportunity for population screening, we must learn from our prior experiences with screening among 50 to 74 year-olds. Achieving high rates of screening requires systems based approaches, such as implementation of mailed FIT outreach,74, 75 and promoting options for choice of screening modality (particularly for racial/ethnic minorities).76–78 Structural racism, poverty, and other factors have led to dramatic disparities in screening observed for those ages 50 to 75 by race, ethnicity, education, and insurance status.1, 79 Disparities and suboptimal screening outcomes are likely to extend to individuals age 45 to 49 unless substantial interventions are resourced to ensure health equity across the entire age-eligible population.80
To optimize early detection and prevention of CRC across the full spectrum of age, we will need to continue to develop precision screening strategies to identify individuals younger than 45 for early screening, implement effective systems based approaches for population-level screening among those ages 45 to 49, and invest substantial resources in increasing screening participation and appropriate follow up for individuals ages 50 to 75 who are not up to date with CRC screening. A multi-pronged approach that acknowledges the importance of the rise in early-onset CRC, but continues to focus resources on optimizing screening for older individuals is critical, because older individuals have CRC risk that is several orders of magnitude higher than those under 50.1
CONCLUSIONS
We continue to make strides in our understanding of early-onset CRC and in the availability of interventions to reduce early-onset CRC morbidity and mortality. Clinician and patient education on early-onset CRC red flag signs and symptoms and options for guidelines-based screening in those who are younger than 50 years of age are key areas of focus that have the potential to begin reducing early-onset CRC mortality rates. We must also continue to identify the factors driving early-onset CRC to inform additional primary prevention approaches, including precision screening, and characterize predictive somatic markers in early-onset CRC to improve treatment outcomes among those with early-onset CRC.
Grant support:
Andrea N. Burnett-Hartman: research support from NCI, R01 CA196337 (Newcomb); U24 CA 221936 (Li/Barlow/Zheng)
Jeffrey K. Lee: research support from NCI, K07 CA212057
Joshua Demb: research support from NCI 5F32 CA239360-02
Samir Gupta: research support from NCI 1UG3CA233314-01A1 (Martínez, Gupta, Casteñeda, MPI); 3 P30
CA023100 (Lippman); 1 R37 CA 222866-01 (Gupta)
Abbreviations:
- CRC
colorectal cancer
- CMS
consensus molecular subtypes
- FIT
fecal immunochemical test
- MSI
microsatellite instability
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose
Contributor Information
Andrea N. Burnett-Hartman, Institute for Health Research, Kaiser Permanente Colorado, Aurora, CO.
Jeffrey K. Lee, Division of Research, Kaiser Permanente Northern California; Department of Gastroenterology, Kaiser Permanente San Francisco Medical Center.
Joshua Demb, Division of Gastroenterology, Department of Internal Medicine, University of California, San Diego, La Jolla, CA, USA.
Samir Gupta, San Diego Healthcare System, San Diego, CA; Division of Gastroenterology, Department of Internal Medicine, University of California, San Diego, La Jolla, CA, USA.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020. [DOI] [PubMed] [Google Scholar]
- 2.Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020;158:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abualkhair WH, Zhou M, Ochoa CO, et al. Geographic and intra-racial disparities in early-onset colorectal cancer in the SEER 18 registries of the United States. Cancer Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009;18:1695–8. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Medhanie GA, Fedewa SA, et al. State Variation in Early-Onset Colorectal Cancer in the United States, 1995–2015. J Natl Cancer Inst 2019;111:1104–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA surgery 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zahnd WE, Gomez SL, Steck SE, et al. Rural-urban and racial/ethnic trends and disparities in early-onset and average-onset colorectal cancer. Cancer 2020. [DOI] [PubMed] [Google Scholar]
- 8.Araghi M, Soerjomataram I, Bardot A, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol 2019;4:511–518. [DOI] [PubMed] [Google Scholar]
- 9.Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, et al. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975–2015. JAMA 2019;321:1933–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019;68:2179–2185. [DOI] [PubMed] [Google Scholar]
- 11.Sung JJY, Chiu HM, Jung KW, et al. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol 2019;114:322–329. [DOI] [PubMed] [Google Scholar]
- 12.Basu P, Ponti A, Anttila A, et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer 2018;142:44–56. [DOI] [PubMed] [Google Scholar]
- 13.Khan NA, Hussain M, ur Rahman A, et al. Dietary Practices, Addictive Behavior and Bowel Habits and Risk of Early Onset Colorectal Cancer: a Case Control Study. Asian Pac J Cancer Prev 2015;16:7967–73. [DOI] [PubMed] [Google Scholar]
- 14.Nimptsch K, Wu K. Is Timing Important? The Role of Diet and Lifestyle during Early Life on Colorectal Neoplasia. Curr Colorectal Cancer Rep 2018;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013;24:335–41. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Jung YS, Park JH, et al. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol 2016;22:3611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen LH, Liu PH, Zheng X, et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr 2018;2:pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol 2019;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old). Clin Gastroenterol Hepatol 2019;17:115–122. [DOI] [PubMed] [Google Scholar]
- 20.Gausman V, Dornblaser D, Anand S, et al. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low EE, Demb J, Liu L, et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banack HR, Bea JW, Kaufman JS, et al. The Effects of Reverse Causality and Selective Attrition on the Relationship Between Body Mass Index and Mortality in Postmenopausal Women. Am J Epidemiol 2019;188:1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Zheng X, Zong X, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng X, Hur J, Nguyen LH, et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J Natl Cancer Inst 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol 2020;17:352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegel RL, Jakubowski CD, Fedewa SA, et al. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book 2020;40:1–14. [DOI] [PubMed] [Google Scholar]
- 27.Losi L, Di Gregorio C, Pedroni M, et al. Molecular genetic alterations and clinical features in early-onset colorectal carcinomas and their role for the recognition of hereditary cancer syndromes. Am J Gastroenterol 2005;100:2280–7. [DOI] [PubMed] [Google Scholar]
- 28.Giráldez MD, Balaguer F, Bujanda L, et al. MSH6 and MUTYH deficiency is a frequent event in early-onset colorectal cancer. Clin Cancer Res 2010;16:5402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol 2017;3:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastrinos F, Samadder NJ, Burt RW. Use of Family History and Genetic Testing to Determine Risk of Colorectal Cancer. Gastroenterology 2020;158:389–403. [DOI] [PubMed] [Google Scholar]
- 31.Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019;125:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieu CH, Golemis EA, Serebriiskii IG, et al. Comprehensive Genomic Landscapes in Early and Later Onset Colorectal Cancer. Clin Cancer Res 2019;25:5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006;38:787–93. [DOI] [PubMed] [Google Scholar]
- 34.Holowatyj AN, Gigic B, Herpel E, et al. Distinct Molecular Phenotype of Sporadic Colorectal Cancers Among Young Patients Based on Multiomics Analysis. Gastroenterology 2020;158:1155–1158.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Q Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol and Toxicol 2013;53:401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coyle C, Cafferty FH, Langley RE. Aspirin and Colorectal Cancer Prevention and Treatment: Is It for Everyone? Curr Colorectal Cancer Rep 2016;12:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nfonsam VN, Jecius H, Chen D, et al. Increasing Incidence of Colon Cancer in the Young: Assessing the Tumor Biology. J Am Coll Surg 2019;229:79–90. [DOI] [PubMed] [Google Scholar]
- 38.Nfonsam VN, Jecius HC, Janda J, et al. Cartilage oligomeric matrix protein (COMP) promotes cell proliferation in early-onset colon cancer tumorigenesis. Surg Endosc 2020;34:3992–3998. [DOI] [PubMed] [Google Scholar]
- 39.Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep 2019;8:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hino H, Shiomi A, Kusuhara M, et al. Clinicopathological and mutational analyses of colorectal cancer with mutations in the POLE gene. Cancer Med 2019;8:4587–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer. Nature 2020;578:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnett-Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer Causes Control 2019;30:747–755. [DOI] [PubMed] [Google Scholar]
- 43.Myers EA, Feingold DL, Forde KA, et al. Colorectal cancer in patients under 50 years of age: A retrospective analysis of two institutions’ experience. World J Gastroenterol 2013;19:5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Read B, Sylla P. Aggressive Colorectal Cancer in the Young. Clin Colon and Rectal Surg 2020;33:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva ACB, Vicentini MFB, Mendoza EZ, et al. Young-age onset colorectal cancer in Brazil: Analysis of incidence, clinical features, and outcomes in a tertiary cancer center. Curr Probl Cancer 2019;43:477–486. [DOI] [PubMed] [Google Scholar]
- 46.Strum WB, Boland CR. Clinical and Genetic Characteristics of Colorectal Cancer in Persons under 50 Years of Age: A Review. Dig Dis and Sci 2019;64:3059–3065. [DOI] [PubMed] [Google Scholar]
- 47.Frostberg E, Rahr HB. Clinical characteristics and a rising incidence of early-onset colorectal cancer in a nationwide cohort of 521 patients aged 18–40 years. Cancer Epidemiol 2020;66. [DOI] [PubMed] [Google Scholar]
- 48.Olivo R, Ratnayake S. Colorectal cancer in young patients: a retrospective cohort study in a single institution. ANZ J Surg 2019;89:905–907. [DOI] [PubMed] [Google Scholar]
- 49.Dharwadkar P, Greenan G, Singal AG, et al. Is Colorectal Cancer in Patients Younger Than 50 Years of Age the Same Disease as in Older Patients? Clin Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 50.Syed AR, Thakkar P, Horne ZD, et al. Old vs new: Risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol 2019;11:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demb J, Liu L, Murphy CC, et al. Young onset colorectal cancer risk among individuals with iron deficiency anemia and hematochezia. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauri G, Sartore-Bianchi A, Russo AG, et al. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen FW, Sundaram V, Chew TA, et al. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin Gastroenterol Hepatol 2017;15:728–737.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott RB, Rangel LE, Osler TM, et al. Rectal cancer in patients under the age of 50 years: the delayed diagnosis. Am J Surg 2016;211:1014–1018. [DOI] [PubMed] [Google Scholar]
- 55.O’Connell JB, Maggard MA, Livingston EH, et al. Colorectal cancer in the young. Am J Surg 2004;187:343–348. [DOI] [PubMed] [Google Scholar]
- 56.Patel SG, Ahnen DJ. Colorectal Cancer in the Young. Current Gastroenterology Reports 2018;20:15. [DOI] [PubMed] [Google Scholar]
- 57.Pasha SF, Shergill A, Acosta RD, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc 2014;79:875–885. [DOI] [PubMed] [Google Scholar]
- 58.Ko CW, Siddique SM, Patel A, et al. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology 2020;159:1085–1094. [DOI] [PubMed] [Google Scholar]
- 59.D’Souza N, Georgiou Delisle T, Chen M, et al. Faecal immunochemical test is superior to symptoms in predicting pathology in patients with suspected colorectal cancer symptoms referred on a 2WW pathway: a diagnostic accuracy study. Gut 2020;0:gutjnl-2020–321956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta S, Bharti B, Ahnen DJ, et al. Potential impact of family history–based screening guidelines on the detection of early-onset colorectal cancer. Cancer 2020;126:3013–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian Y, Kharazmi E, Brenner H, et al. Calculating the Starting Age for Screening in Relatives of Patients With Colorectal Cancer Based on Data From Large Nationwide Data Sets. Gastroenterology 2020;159:159–168.e3. [DOI] [PubMed] [Google Scholar]
- 62.Jeon J, Du M, Schoen RE, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology 2018;154:2152–2164.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Archambault AN, Su YR, Jeon J, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants is More Strongly Associated With Early-onset vs Late-onset Cancer. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson DJ, Ladabaum U. Opportunities and Challenges in Moving From Current Guidelines to Personalized Colorectal Cancer Screening. Gastroenterology 2019;156:904–917. [DOI] [PubMed] [Google Scholar]
- 65.U.S. Preventive Services Task Force. Colorectal Cancer: Screening (Draft Recommendation Statement). 2020. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3
- 66.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018. [DOI] [PubMed] [Google Scholar]
- 67.Knudsen AB, Rutter CM, Peterse EFP, et al. Colorectal Cancer Screening: A Decision analysis for the US Preventive Services Task Force. AHRQ Publication No.20-05271-EF-2, 2020. [Google Scholar]
- 68.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018;124:2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butterly LF, Siegel RL, Fedewa S, et al. Colonoscopy Outcomes in Average-Risk Screening Equivalent Young Adults: Data From the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2020. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Levin TR, Jensen CD, Chawla NM, et al. Early Screening of African Americans (45–50 Years Old) in a Fecal Immunochemical Test-Based Colorectal Cancer Screening Program. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology 2019;157:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly JJ, Alberts SR, Sacco F, et al. Colorectal cancer in alaska native people, 2005–2009. Gastrointest Cancer Res 2012;5:149–54. [PMC free article] [PubMed] [Google Scholar]
- 73.Paquette IM, Ying J, Shah SA, et al. African Americans should be screened at an earlier age for colorectal cancer. Gastrointest Endosc 2015;82:878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levin TR, Corley DA, Jensen CD, et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018;155:1383–1391.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta S, Coronado GD, Argenbright K, et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: Summary of a Centers for Disease Control and Prevention-sponsored Summit. CA Cancer J Clin 2020;70:283–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013;173:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singal AG, Gupta S, Skinner CS, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. JAMA 2017;318:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.May FP, Yang L, Corona E, et al. Disparities in Colorectal Cancer Screening in the United States Before and After Implementation of the Affordable Care Act. Clin Gastroenterol Hepatol 2020;18:1796–1804.e2. [DOI] [PubMed] [Google Scholar]
- 80.Demb J, Gupta S. Racial and Ethnic Disparities in Colorectal Cancer Screening Pose Persistent Challenges to Health Equity. Clin Gastroenterol Hepatol 2020;18:1691–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]


