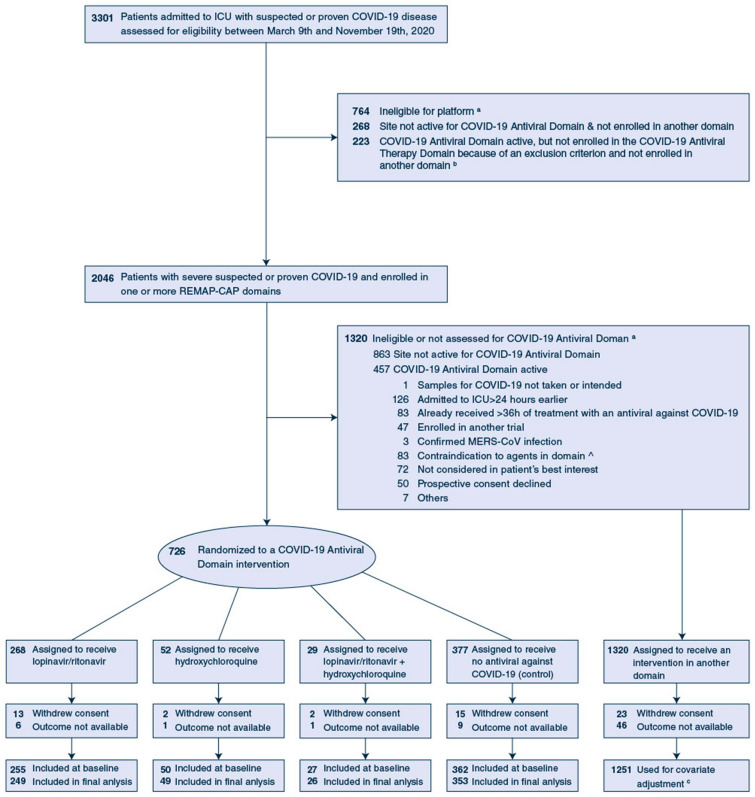
Fig. 1.
Screening, randomization, and follow-up of patients in the REMAP-CAP COVID-19 Antiviral Therapy Domain randomized controlled trial. aPatients could meet more than one ineligibility criterion (Table S2, Supplementary Appendix). bDetails of platform exclusions are provided in the Supplementary Results (Supplementary Appendix). cThe primary analysis of organ support-free days (OSFD) and hospital survival were conducted on the REMAP-CAP intention-to-treat cohort which included all patients enrolled in the trial who met COVID-19 severe state criteria and were randomized within at least one domain, adjusting for patient factors and for assignment to interventions in other domains (Table S3, Supplementary Appendix). ^Contraindications include hypersensitivity, receiving the study drug as usual medication prior to hospitalization, human immune deficiency (HIV) infection (contraindication of lopinavir-ritonavir), severe liver failure (contraindication of lopinavir-ritonavir), receiving amiodarone as a usual medication prior to this hospitalization or any administration of amiodarone within the 72 h prior to assessment (contraindication of lopinavir-ritonavir) and high clinical risk of sustained ventricular dysrhythmia (contraindication of hydroxychloroquine) (Table S2, Supplementary Appendix)