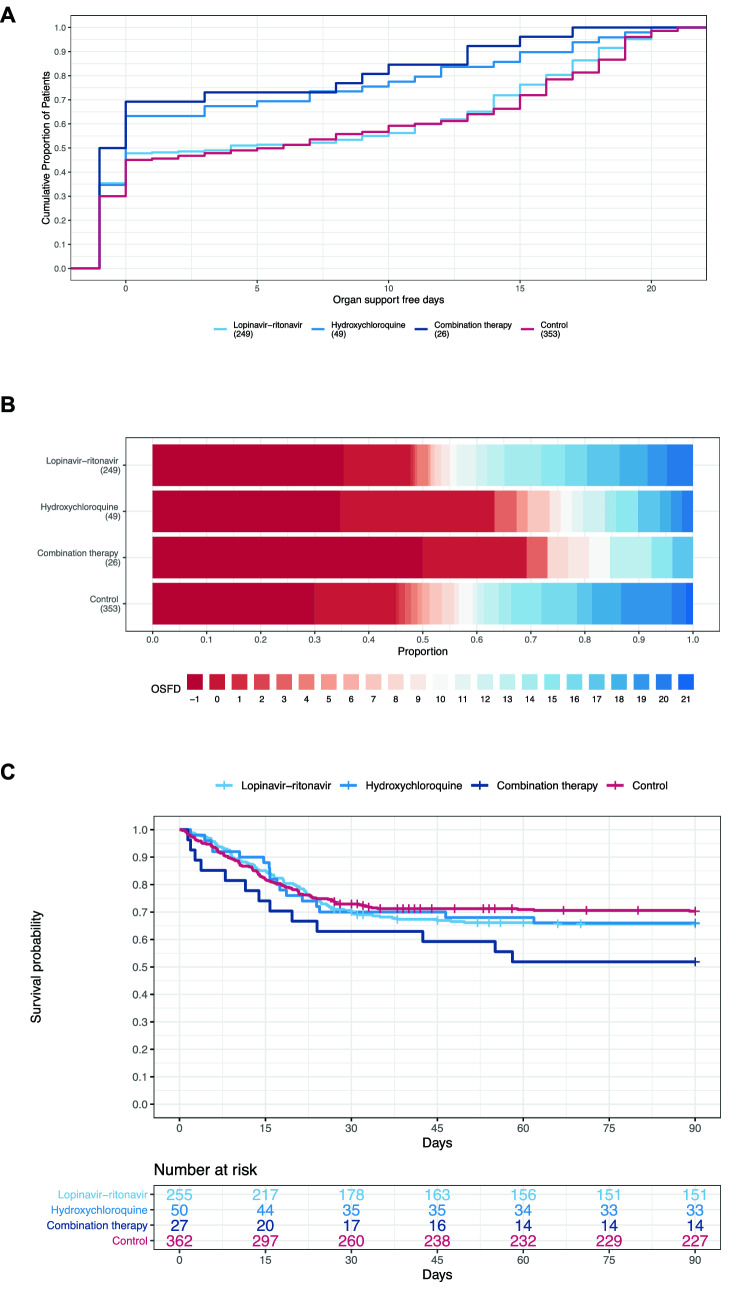
Fig. 2.
Organ support-free days and mortality. A Organ support-free days in patients allocated to lopinavir-ritonavir, hydroxychloroquine, combination therapy and control among critically ill patients in the COVID-19 Antiviral Therapy Domain of the REMAP-CAP trial. Distributions of organ support-free days are displayed as the cumulative proportion (y axis) for each study group by day (x axis). Curves that rise more slowly are more favorable. The height of each curve at “ − 1” indicates the in‐hospital mortality for each intervention. The height of each curve at any time point indicates the proportion of patients who had that number of organ support-free days or fewer. The difference in the height of the curves at any point represents the difference in the percentile in the distribution of organ support-free days associated with that number of days alive and free of organ support. B Organ support-free days are displayed as horizontally stacked proportions by study group. Red represents worse values and blue represents better values. On primary analysis of organ support-free days, the three interventions decreased organ support-free days compared to control, with corresponding median adjusted ORs and 95% credible intervals of 0.73 (0.55–0.99), 0.57 (0.35–0.83) and 0.41 (0.24–0.72), respectively, yielding high posterior probabilities of futility (99% or greater) and high posterior probabilities of harm compared to control (98%, 99.9% and > 99.9%, respectively). C Empirical distribution of survival for lopinavir–ritonavir, hydroxychloroquine, combination therapy and control. Lopinavir-ritonavir, hydroxychloroquine and combination therapy resulted in reduced survival over 90 days compared to control, with adjusted median hazard ratios (95% CrI) of 0.83 (0.65, 1.07), 0.71 (0.45, 0.97), 0.58 (0.36, 0.92), yielding high probabilities of harm compared with control of 92% and 98.4% and 98.7%, respectively