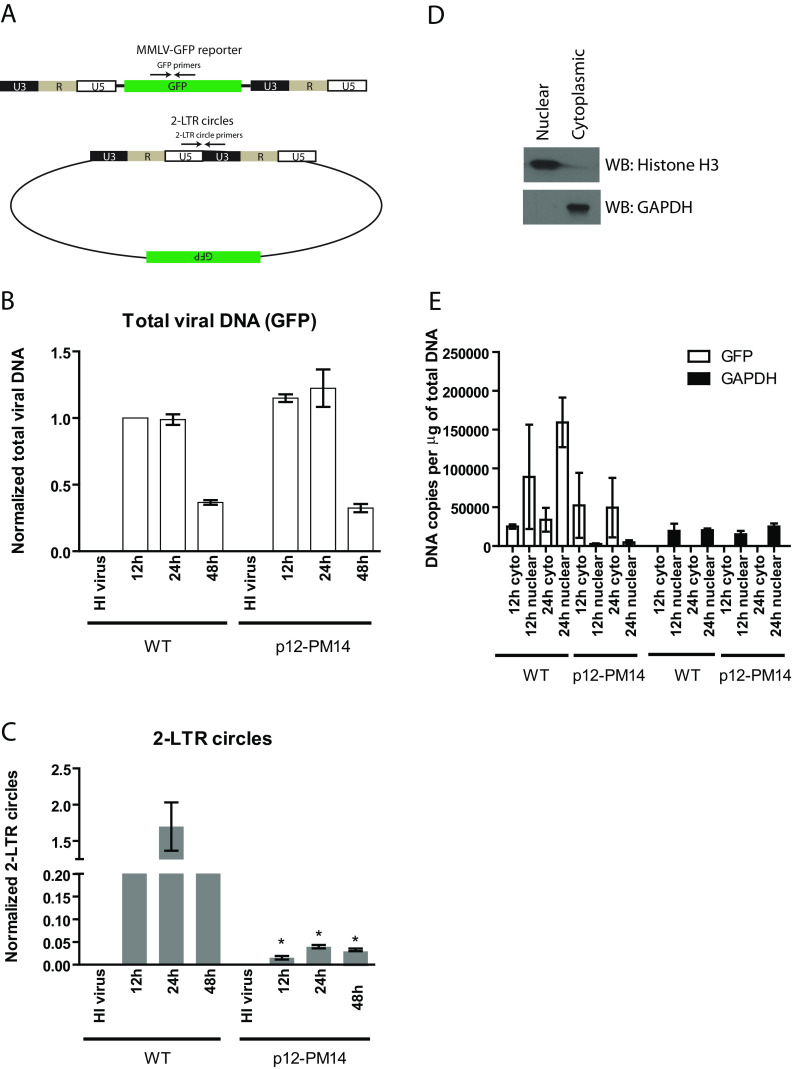
FIG 1.
MMLV p12 mutant defective in chromatin tethering has normal DNA synthesis but defective nuclear entry. (A) Schematic of MMLV single-round GFP reporter virus DNA and 2-LTR circular DNA generated during infection. Locations of PCR primers targeting GFP and 2-LTR circles are shown. (B) HeLa cells were infected with VSV-G pseudotyped wild-type (WT) or p12-PM14 mutant MMLV-GFP viruses. Total DNA were isolated from infected cells at different time points, followed by real-time quantitative PCR using primers targeting GFP (total viral DNA). Levels of total viral DNA were first normalized using the 2−ΔΔCT method to the value obtained for the GAPDH gene; the obtained values were then normalized to the values obtained from HeLa cells infected with WT virus at 12 h (set to 1). To control for potential plasmid DNA carryover in the viral supernatant, heat-inactivated (HI) virus was used in parallel. Results shown are means ± standard errors (SEs) from three independent experiments. (C) Similar experiment as that in panel B using primers targeting 2-LTR circles. Student’s t test was used for statistical analysis. *, P < 0.05 compared to the same time point in WT virus. (D) Nuclear or cytoplasmic extract prepared from HeLa cells was subjected to Western blotting with anti-histone H3 (nuclear marker) or anti-GAPDH (cytoplasmic marker). (E) HeLa cells infected with VSV-G pseudotyped WT or p12-PM14 mutant MMLV-GFP viruses were subjected to nuclear/cytoplasmic fractionation at 12 and 24 h postinfection. Total DNA were then isolated from cellular fractions followed by real-time qPCR using primers targeting GFP (total viral DNA) or GAPDH. The absolute copy number per μg of input DNA is calculated based on standard curves generated using plasmid DNA. The results shown are means ± standard deviations (SDs) from two independent experiments performed in duplicate.