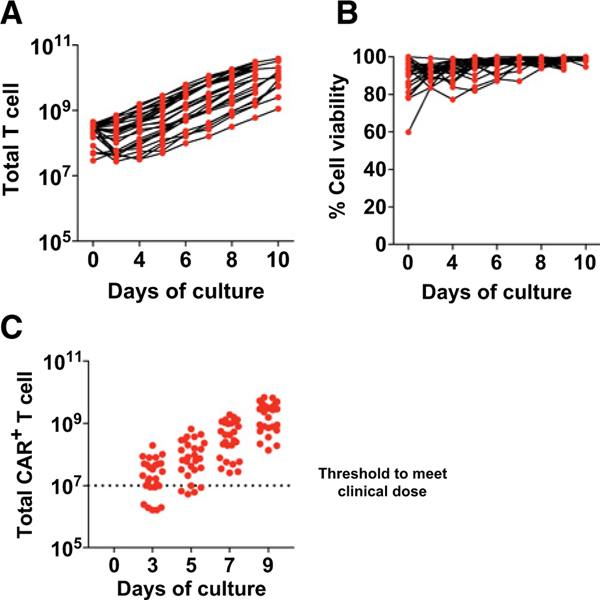
Figure 4.
An abbreviated CAR T culture approach is technically feasible in a GMP large-scale production facility. A, T cells from ALL patients were stimulated with anti-CD3/CD28 microbeads and expanded ex vivo using a large-scale cGMP process as described in the Materials and Methods. Activated T cells were transduced with CD19-BBζ CAR. Total T-cell number during expansion is shown (n = 27 independent patients).B, T-cell viability was assessed by flow cytometry over time for the patients in A. C, CD19-CAR abundance was measured by qPCR at days 3, 5, 7, and 9 of expansion. The target dose of day 9 CAR+ T cells for clinical applications is represented by the dotted line.