Abstract
Background
Particulate matter (PM), a major component of ambient air pollution, accounts for a substantial burden of diseases and fatality worldwide. Maternal exposure to PM during pregnancy is particularly harmful to children’s health since this is a phase of rapid human growth and development.
Method
In this review, we synthesize the scientific evidence on adverse health outcomes in children following prenatal exposure to the smallest toxic components, fine (PM2.5) and ultrafine (PM0.1) PM. We highlight the established and emerging findings from epidemiologic studies and experimental models.
Results
Maternal exposure to fine and ultrafine PM directly and indirectly yields numerous adverse birth outcomes and impacts on children’s respiratory systems, immune status, brain development, and cardiometabolic health. The biological mechanisms underlying adverse effects include direct placental translocation of ultrafine particles, placental and systemic maternal oxidative stress and inflammation elicited by both fine and ultrafine PM, epigenetic changes, and potential endocrine effects that influence long-term health.
Conclusion
Policies to reduce maternal exposure and health consequences in children should be a high priority. PM2.5 levels are regulated, yet it is recognized that minority and low socioeconomic status groups experience disproportionate exposures. Moreover, PM0.1 levels are not routinely measured or currently regulated. Consequently, preventive strategies that inform neighborhood/regional planning and clinical/nutritional recommendations are needed to mitigate maternal exposure and ultimately protect children’s health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12199-021-00995-5.
Keywords: Air pollution, Particulate matter, PM2.5, Ultrafine particles, Prenatal exposure, Children’s environmental health, Health effects
Background
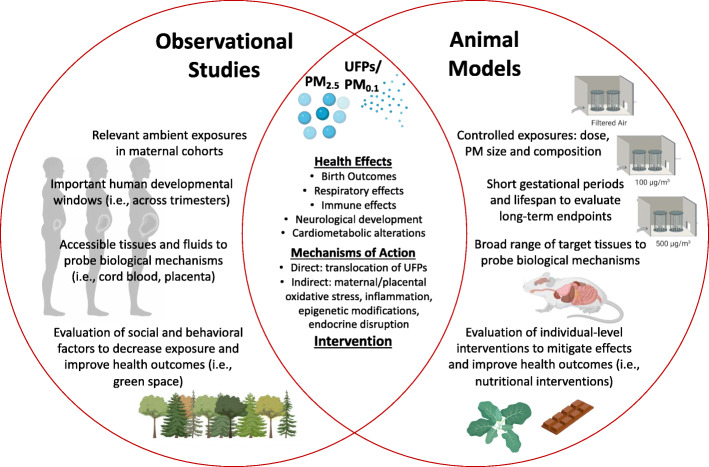
Human exposure to ambient air pollution is a pervasive public health issue based on the substantial cause of disease and death worldwide [1]. Suspended aerosols known as particulate matter (PM) are a predominant toxic component of ambient air pollution emitted by a variety of sources, including vehicular traffic, coal-burning power plants, waste burning, and other industrial activities. Particulate matter (PM) is classified by size as either “coarse” (PM10) with an aerodynamic diameter less than 10 μm, “fine” (PM2.5) with a diameter less than 2.5 μm, or “ultrafine” (PM0.1) with a diameter less than 0.1 μm. The fine and ultrafine fractions can penetrate deeper in the airways in comparison to coarse particles, leading to numerous adverse health effects. A wealth of evidence highlights maternal exposure to pollutants during pregnancy represents a window of susceptibility for fetal development and children’s long-term health. For instance, it is well-established that early life tobacco smoke exposure increases the risk of respiratory infection and asthma in infancy and childhood [2, 3]. Analogous to tobacco smoke, developmental exposure to PM2.5 has been intensively investigated in human epidemiological studies. Outcomes at birth and early respiratory effects are extensively reviewed elsewhere [4–6]. In this review, we will provide an overview of these findings, including emerging data related to ultrafine PM exposure. Additionally, data on neurological effects and cardiometabolic disease risk continue to emerge, and key findings are highlighted [7–9]. Laboratory-based inhalation toxicology studies using in vivo models are also instrumental in establishing causality between prenatal fine and ultrafine PM exposure and adverse outcomes observed in human populations. A main objective of this review was to comprehensively examine experimental models evaluating early life exposure to PM2.5 and/or PM0.1 and effects on offspring. Additionally, we summarize the evidence on underlying mechanisms of action gleaned from human and nonhuman studies. Last, we briefly summarize preventive intervention strategies related to mitigating maternal exposure, but detailed behavioral, neighborhood, and nutritional interventions is beyond the scope of this review. Overall, we highlight the evidence gleaned from human observational studies and animal models is essential in characterizing adverse health outcomes and deciphering the mechanisms of action to support preventive strategies to ultimately protect children’s health (Fig. 1).
Fig. 1.
Evidence gleaned from human observational studies and animal models is essential in characterizing adverse health outcomes and deciphering the mechanisms of action to support preventive intervention testing. Selected strengths of these two methodological approaches are highlighted in the Venn diagram. In this review, we synthesize the scientific evidence on adverse health outcomes in children following prenatal exposure to fine particulate matter (PM2.5) and ultrafine particles (UFPs, PM0.1). Additionally, we summarize the evidence on underlying mechanisms of action. Created with BioRender.com
Study selection
Five literature searches were performed using PubMed (details in Supplemental file). The search strategy combined key sets of words using AND/OR. In the first search, terms included “prenatal” AND “particulate matter” OR “ultrafine.” Two independent reviewers (NMJ and an additional content author) scanned titles and abstracts to determine eligibility and to further categorize studies into human or nonhuman evidence. Subsequently, studies were categorized by endpoints, such as birth outcomes, respiratory/immune, brain, and cardiometabolic. We included original studies that evaluated prenatal exposure to ambient fine or ultrafine PM. We excluded studies if (1) the article did not report ambient PM2.5 or PM0.1 exposure; (2) the article contained no original data related to human on nonhuman (rodent) health effects in each pre-defined endpoint category; or (3) other reason, with an explanation required. All duplicate articles were removed. Two independent reviewers reviewed the full-text articles for inclusion and excluded studies that were duplicates, nonrodent, or not related to their primary endpoint. Four additional searches were carried out the same as above using 1: “particulate matter” AND “pregnancy” AND “oxidative stress”; 2: “particulate matter” AND “pregnancy” and “inflammation”; 3: “particulate matter” AND “pregnancy” AND “epigenetic”; and 4: “particulate matter” AND “pregnancy” AND “endocrine.”
Human evidence from epidemiologic studies
Adverse birth outcomes
There is a strong body of human evidence from epidemiological studies associating PM, in particular PM2.5, with adverse birth outcomes. Systematic reviews with meta-analyses [10–13], summarized in Table 1, demonstrate positive, and often significant, associations between PM2.5 exposure across the entire pregnancy and increased risk of preterm birth and infant low birth weight. Effect estimates for every 10 μg/m3 increase in PM2.5 exhibit similar ranges across analyses for reductions in birth weight from −15.9 to −23.4 g. Studies have also interrogated the impact of exposure during specific trimesters to identify critical windows of susceptibility. Overall, the results are mixed; however, a common theme of increased impact appears for exposure later in pregnancy. DeFranco et al. [14] also observed the greatest risk for preterm birth (19% increased risk) in the third trimester. Percy et al. [15] demonstrated increasing exposure to PM2.5 during the third trimester, in particular between 30 and 35 weeks of gestation, was associated with an increased risk for small for gestational age babies. In an observational natural experiment, infants whose 8th month of gestation occurred during the 2008 Beijing Olympics, when air pollution levels drastically deceased, were born 23 g heavier on average compared to infants whose 8th month occurred over the same dates the year prior (2007) or after (2009) [16]. It is plausible exposure later in pregnancy, during periods of rapid fetal weight gain, has a larger impact on infant birth weight. Likewise, stressors closer to delivery could affect preterm birth. However, for other adverse outcomes, such as cognitive effects, earlier exposure during neurogenesis may be considered the critical window. Continued research incorporating smaller windows, such as weeks, may help further illuminate critical windows of susceptibility.
Table 1.
Summary of meta-analyses results related to preterm birth or infant low birth weight and PM2.5 exposure across pregnancy
| Reference | Odds ratio for preterm birth (95% CI) | Odds ratio for low birth weight (95% CI) | Effect estimate (g) for every10 μg/m3 increase in PM2.5 |
|---|---|---|---|
| Stieb et al. [10] | 1.05 (0.98, 1.13) n = 4 | 1.05 (0.99, 1.12) n = 6 | −23.4 (−45.5, −1.4) n = 7 |
| Zhu et al. [11] | 1.10 (1.03, 1.18) n = 8 | 1.05 (1.02, 1.07) n = 6 | −14.6 (−19.3, −9.9) n = 12 |
| Lamichhane et al. [12] | 1.13 (0.98, 1.28) n = 5 | NA | −22.2 (−37.9, −6.4) n = 7 |
| Sun et al. [13] | NA | 1.09 (1.03, 1.15) n = 19 | −15.9 (−26.8, −5.0) n = 17 |
The risk of stillbirth has also been investigated in association with prenatal exposure to PM2.5. A meta-analysis including 13 studies cited exposure to ambient air pollution increases the risk of stillbirth; however, PM2.5 alone was neither found to be statistically significant [17], nor was it significant in the meta-analysis performed by Zhu et al. [11]. Similarly, in an Ohio cohort, “high” PM2.5 exposure, defined as greater than or equal to the mean PM2.5 level during the study period (13.3 μg/m3) plus the IQR for the specific time period measured for each birth) was not associated with increase in stillbirth risk through pregnancy, first or second trimester; however, high PM2.5 exposure during the third trimester was associated with 42% increased stillbirth risk [18]. Further epidemiological and mechanistic studies are needed to validate the causal linkage between PM2.5 exposure and stillbirth risk.
Respiratory effects and impact on the immune system
Prenatal exposure to PM2.5 impacts lung development and respiratory health in a variety of ways that may persist throughout childhood [5]. Developmental PM2.5 exposure can lead to disturbed alveolarization, impaired lung function, and pulmonary immune differentiation, which may influence acute and chronic health outcomes. In a meta-analysis of multiple European birth cohorts, MacIntyre et al. [19] concluded there was consistent evidence for an association between air pollution and pneumonia in early childhood. The link between prenatal PM2.5 exposure and the development of asthma has also become increasingly recognized as epidemiologic studies have reported positive associations [6, 20]. Hehua et al. [6] reviewed 18 studies and found that children prenatally exposed to multiple air pollutants had increased risk of wheeze and asthma during childhood. However, only a weak association was identified in the five existing studies that evaluated prenatal PM2.5 exposure. These findings highlight a research gap and the need for future studies to examine PM2.5 exposure and contribution to asthma etiology, as well as mechanistic studies to tease apart the complex gene-environment interactions involved in asthma development.
PM2.5 also affects the immune system, although only a handful of human studies relevant to early life exposure are published to date [21, 22]. Herr et al. [23] observed that prenatal exposure to PM2.5 shifted lymphocyte distributions representative of the neonatal adaptive immune response in umbilical cord blood. Exposure during early gestation resulted in increased T lymphocytes, decreased B lymphocytes, and natural killer cells, whereas late gestation exposure was associated with an opposite immune phenotype with decreased T lymphocytes, increased B lymphocytes, and natural killer cells. Since infants are at risk for respiratory infections, the impact of PM2.5 on neonatal immunity may significantly influence morbidity risk [24].
Effects on neurological development
The importance of cognitive function and increasing prevalence of neurodevelopment disorders, including developmental delay, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorders (ASD) has spurred a large amount of research examining early life exposure to ambient air pollution [7, 25, 26]. In general, epidemiological studies have investigated the impact of prenatal exposure to PM2.5 on structural alterations, cognitive function, and risk of clinically defined disorders. In a study examining prenatal PM2.5 exposure, children presented with thin cortex in many regions of the brain and an impaired inhibitory control. Impaired inhibitory control is related to other mental health problems, including addictive behavior and ADHD [27]. Mortamais et al. [28] estimated prenatal PM2.5 levels at maternal residential addresses during the 3rd trimester of pregnancy and observed an increase of 7 μg/m3 was significantly associated with a decreased corpus collosum (the bridge connecting the two hemispheres) body volume. This decreased volume was linked with higher hyperactivity scores, indicative of behavioral issues. Prenatal PM2.5 exposure has also been correlated with reduced fundamental cognitive abilities, including working memory and conflict attentional network [29]. In a systematic review of multiple airborne pollutants and ASD risk, Lam et al. [26] concluded the strongest evidence was between prenatal exposure to PM2.5. However, the small number of studies (n = 3) in the meta-analysis and unexplained heterogeneity signified that the effect could be larger or smaller than those studies estimate, supporting the need for additional research on PM2.5 exposure and ASD risk. Overall, the mounting human evidence suggests developmental PM2.5 exposure effects neurobehavioral function and contributes to cognitive impairment. Advancing air pollution policy for the protection of children’s health and promotion of healthy brains, similar to prevention of lead poisoning and mitigation of cognitive deficits, is warranted on the basis of this burgeoning epidemiological evidence [9].
Metabolic alterations
In the USA, the prevalence of childhood obesity has nearly doubled since 2000 [30]. Over 18 million children are obese, 19% of the population in 2015-2016, in comparison to less than 10% of the population in 1999-2000. Parallel to this increase in obesity is an elevated prevalence of type 2 diabetes [31]. While the etiology of type 2 diabetes is multi-factorial, obesity is a primary risk factor as this state disrupts insulin homeostasis leading to abnormal blood glucose levels [32]. Studies looking at early life environmental influences on the development of metabolic disorders have emerged in the face of the current obesity epidemic [33]. A handful of studies have evaluated how developmental air pollution exposure impacts offspring body mass and metabolic disease risk. Alderete et al. [34] demonstrated that prenatal residential traffic-related air pollution exposure was associated with higher adipokines, leptin, and adiponectin levels in umbilical cord blood. Increased leptin levels correlated with significant weight gain in female infants, which investigators reasoned could increase future obesity risk. In subsequent work, researchers observed higher NO2 and PM2.5 levels were correlated with altered β cell function and insulin sensitivity and was associated with a higher body mass index (BMI) at 18 years of age [35]. In another study, infants exposed to higher traffic-related air pollution in early life were more likely to develop the “thrifty phenotype,” where infants initially born with low birth weight were more prone to rapid weight gain in the first 6 months of life [36]. Specific to prenatal PM2.5 exposure in the 3rd trimester, effect estimates were in the same direction, but smaller and imprecise. The same investigators followed a cohort of 1400 children that lived close to a highway during delivery. Children were found to have increased adipose tissue accumulation during early and mid-childhood [37]. Paradoxically, PM2.5 exposure improved cardiometabolic markers. In a follow-up study, closer residential proximity to freeways was associated with a higher BMI in early childhood, but showed no evidence of a persistent effect [38]. In a German cohort, Thiering et al. [39] showed insulin resistance was greater in children at 10 years of age who were exposed early in life to traffic-related air pollution. In a follow-up study, landscape “greenness” attenuated the effect, which could be attributable to lower pollution exposure [40]. Moody et al. [41] observed prenatal and perinatal PM2.5 exposure was associated with changes in HbA1c levels in early childhood. This marker is indicative of glucose dysregulation, providing evidence that early life exposure may influence diabetes risk. Another study demonstrated that third trimester PM2.5 exposure led to increased risk of offspring high blood pressure, which could further lead to cardiometabolic dysfunctions [42]. Complicating research on hypertensive disorders, gestational hypertension is associated with higher offspring blood pressure; several studies show maternal PM2.5 exposure may increase the risk of gestational hypertension or preeclampsia, a risky pregnancy complication characterized by high blood pressure [43–45]. In summary, additional research is needed on how PM2.5 exposure during pregnancy leads to metabolic alterations in children later in life.
Summary of human evidence
Based on the numerous epidemiological studies summarized above, it is clear that prenatal exposure to fine PM is associated with numerous adverse effects in children, including acute birth outcomes and chronic respiratory effects, with a growing body of literature indicating cognitive and metabolic dysfunction. Although not currently regulated through air quality standards, ultrafine particles (UFPs, PM0.1) are postulated to exert enhanced toxicities due to their larger surface area/mass ratio, enhanced oxidative capacity and ability to translocate into systemic circulation [46]. In general, there is a lack of human evidence on the specific effects from prenatal exposure to UFPs, in part due to the lack of monitoring and models to estimate UFP exposure [47]. In the first large-scale epidemiologic study, Lavigne et al. [48] demonstrated in addition to PM2.5 and nitrate exposure, prenatal UFP exposure was independently associated with childhood asthma incidence. Wright et al. [49] also observed prenatal UFP exposure was associated with asthma development in children in the Northeastern USA, independent of NO2 and temperature. These emerging findings emphasize the need to fill the current gap in the literature interrogating the relationship between prenatal UFP exposure and adverse health outcomes in offspring, particularly immune, neurological, and cardiometabolic endpoints. Further data will help verify the independent risk of UFP exposure on developmental endpoints, as well as inform the effects from multi-pollutant models. In the scarcity of human evidence, a variety of recent studies in animal models have investigated the specific effects of UFP exposure. Described in more detail in the next section, phenotypic and mechanistic data gleaned from animal models help support improved knowledge and encourage further regulation of air pollution exposure during this critical window of development.
Nonhuman evidence from experimental models
Experimental approaches
Animal models provide controlled exposure conditions across defined developmental periods to aid in determining dose-response gradients and biological mechanisms of action (Fig. 1). Rodent models, both rats and mice, have been extensively employed to research the effects of prenatal fine and ultrafine PM exposure on offspring. Short gestational windows (approximately 19-21 days for mice and 21-23 days for rats) and rapid offspring development to maturity allow investigators to conduct experiments relatively quick in comparison to human cohort studies that require years before developmental endpoints can be assessed. The broad term “early life exposure” is commonly used in the human and nonhuman literature to indicate prenatal, neonatal, and perhaps childhood exposure. The following section summarizing the nonhuman evidence from rodent exposure models defines the prenatal window as exposure occurring in utero, i.e., during gestation. Sometimes, investigators design experimental studies to encompass the perinatal period and employ exposures after birth. This is based on developmental endpoints, since rodent exposures in early infancy (i.e., the neonatal period) mimic human 3rd trimester exposures for several organs, including the lung [50] and brain [51]. In reality, human exposure can occur before, during, and after pregnancy, and animal models allow researchers to control the precise timing of exposures, which helps define critical windows of susceptibility.
In addition to controlling timing of exposure in models, researchers can regulate the size and dose of PM. Methods for generating and characterizing PM in inhalation toxicology models are reviewed in detail by Chen and Lippmann [52]. In general, models of pre- and perinatal exposures have employed various agents (i.e., components of PM) and particle sizes (fine and/or ultrafine fractions) using different routes, most frequently inhalation not only via whole-body or nose-only chambers but also intranasal and intratracheal instillation in some cases. Stress induced from confinement within nose-only chambers and the anesthesia required for particle instillation are limitations of particular importance when working with pregnant dams. A few investigators have utilized ambient air exposures through direct exposure to traffic or polluted urban air [53, 54]. Direct exposure to traffic and ambient urban air can vary temporally, thus, may limit reproducibility. However, a strength is environmentally relevant atmospheric concentrations. For instance, exposures have averaged around 16.8 ± 8.3 (SD) μg/m3 in São Paulo [53] to 73.5 ± 61.3 (SD) μg/m3 in Beijing [54].
Several research teams have utilized concentrated ambient particulate matter systems (CAPs) to deliver fine and ultrafine PM mixtures to pregnant mice and neonates [52]. One example is the Harvard University Concentrated Ambient Particle System (HUCAPS) fitted with a size-selective inlet for ultrafine particles (< 100 nm) applied in numerous early life exposure models [55–60]. The HUCAPS system concentrates ultrafine particles approximately ten times that of ambient air concentrations. The gas-phase components of the ambient aerosol are present but are not concentrated by the system. PM levels generated have averaged around 67.9 μg/m3 (1.82 × 105 particles/m3) to 96.4 μg/m3 (2.02 × 105 particles/m3), reflecting particle numbers and concentrations reported in U.S. cities [55]. Additionally, studies have been carried out using comparable systems, such as the New York University Versatile Ambient Particle Concentrator Exposure System (VACES) [61–63] and the Ohio State University OASIS-1 aerosol concentration system [64–66]. The VACES system concentrates ambient PM at an equivalent factor to the HUCAPS system, with a slightly larger particle size distribution within the fine and ultrafine range. Using the VACES system, Klocke et al. [61] generated an average concentration of 92.69 ± 19.16 (SD) μg/m3, and Church et al. [63] produced average levels of 135.8 ± 13.17 μg/m3, representing 11 times ambient air concentrations. Similar average levels were recorded for concentrated PM2.5 from the Columbus, OH region [64–66]. Overall, these systems have generated human relevant exposures for experimental testing.
In addition to concentrated ambient particulate matter systems, aerosolizing PM from a defined source represents a complementary approach refined in its ability to generate consistent daily PM concentrations from distinct sources. Zhang and colleagues generated an ultrafine PM mixture from a multicomponent aerosol mixture representative of PM chemical composition under typical polluted urban environments [67] using an atomizer and diluted solution consisting of organics, sulfates, nitrates, ammonium, chloride, and diesel exhaust PM [68]. Particle size ranged from 20 to 220 nm, with a peak diameter of 50 nm. Using this system, Rychlik et al. [68] reported an average mass concentration of 101.94 μg/m3, corresponding to a 24-h daily mean dose of 25 μg/m3. Notably, while there is no current regulatory standard for ultrafine PM, this level is under the U.S. EPA national ambient air quality standard of 35 μg/m3 for PM2.5 and similar to the WHO recommended guideline of 25 μg/m3 for 24-h average exposure. Using the same system, Wu et al. [69] investigated the impact of ultrafine ammonium sulfate particles (peak diameter of 10-20 nm) on offspring development. This exposure replicated several key PM properties observed during polluted haze events in Asia, including chemical composition, size, hygroscopicity, and acidity [70]. Cormier and colleagues have also investigated the specific toxic effects of combustion-generated environmentally persistent-free radicals (EPFR) [71] adsorbed to ultrafine particles in several exposure models [72–76]. EPFRs denote integrated pollutant-particle systems consisting of phenoxyl- and semiquinone-type radicals formed and stabilized by transition metal oxide–containing particles. These represent exposures identified in airborne PM at abandoned hazardous waste facilities that fall under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, termed Superfund sites [77]. These in vivo inhalation models mimic environmentally relevant doses (200 μg/m3), providing an alveolar deposition dose to neonates equivalent to deposition in human infants [73]. Other research groups have applied similar approaches to probe the effects of exposure to fossil fuel combustibles, such as aerosolized residual oil fly ash [78] and vehicular-derived PM [79–81]. In several models, Morgan and colleagues have applied re-aerosolized nano-scale PM (< 200 nm) collected from a heavy traffic site nearby the Los Angeles I-110 Freeway using a high-volume ultrafine particle sampler [79, 80]. Researchers have also re-aerosolized diesel exhaust particles (DEPs) in numerous in vivo inhalation models using engine-produced particles [82], including National Institute of Standards and Technology (NIST) standard reference materials [81].
Alternatively, other groups have applied DEPs in prenatal exposure models via intranasal application [83, 84], oropharyngeal administration [85], and intratracheal instillation [86, 87]. Outcomes from instillation and inhalation exposures can result in differing pathological consequences [88]; however, in the case of in utero exposures, translocation of the particles into systemic circulation may be of greater consequence to fetal development. Investigators have taken care to apply relevant exposure concentrations using these techniques. For instance, Chen et al. [87] applied 20 μg of a DEP suspension, representing an average daily dose of 8.6 μg/mouse, approximately equating to an inhalational exposure level of 160 μg/m3 PM2.5. Instillation has also been used in general for PM2.5 [89, 90] and PM0.1 [91, 92] dosing using particles collected in urban environments. Oral gavage [93] and intraperitoneal (i.p.) injection of particles [94] are much less commonly used; however, authors cite technical advantages in comparison to intratracheal instillation. While systemic administration of particles does not represent a physiology route of exposure [95], translocation into systemic and placenta circulation may serve as a proxy to investigate fetal/offspring effects. Last, traffic-related air pollution has been extensively studied in animal models via direct exposure to freshly generated diesel exhaust (DE) [96]. Here, rodents are exposed to both particulate and gaseous components. In several prenatal mouse exposure models, DE concentrations have ranged from 90 to 300 μg/m3 PM2.5 [97–100]. Overall, the many inhalation toxicology methods for generating controlled PM exposures has spurred substantial research in rodent models demonstrating effects on offspring respiratory, immune, neurological, and cardiometabolic development (described in detail in this section). These findings bolster the results gleaned from human epidemiological studies and provide a deeper understanding of the underlying biological mechanisms.
Developmental effects
Studies conducted in rodent models have demonstrated varying degrees of adverse birth outcomes following developmental exposure to PM (Table 2). Some models report no effects on outcomes, such as abortion, stillbirth, intrauterine growth restriction, or impact on birth weight. Limitations are associated with assessing some early outcomes, such as stillbirth, as well as differences in models, including species, strain, timing, and type of exposure. There is broad consensus on initial pollutant-induced growth restriction, with more variation in the long-term effects of altered offspring growth trajectories that is driven by differences in exposure models.
Table 2.
Summary of developmental effects from in vivo models (7 studies)
| Reference | Animal model | PM source | Dose | Route | Duration | Offspring effects |
|---|---|---|---|---|---|---|
| Tsukue et al. [101] | C57BL/6J mice | Diesel exhaust | 0.3, 1.0, or 3.0 mg DEP/m3 | Inhalation | 4 months pre-mating exposure (12 h/day 7 days/week) | Decreased BW in both sexes; AGD lengths shorter; organ weights less, and vaginal orifices of young females opened significantly earlier (exposed to 0.3 and 1.0 mg DEP/m3) |
| Hougaard et al. [81] | C57BL/6J mice | Diesel exhaust particles | ~19 mg/m3 | Inhalation | GD9–GD19 (1 h/day) | Decreased weight gain during lactation; cognitive function and biomarkers were generally similar across offspring |
| Gorr et al. [64] | FVB mice | PM2.5 | 51.69 μg/m3 | Inhalation | Gestation/nursing (6 h/day, 7 days/week in utero until weaning at 3 weeks of age) | Reduced birth weight; at adulthood: reduced left ventricular fractional shortening; reduced ejection fraction; increased end-systolic volume; and reduced dP/dt maximum and minimum; alerted cardiomyocytes profiles; increased collagen deposition |
| Liu et al. [89] | Sprague Dawley rats | PM2.5 | 15 mg/kg | Intratracheal | GD10 and GD18 | Increased absorbed blastocysts; lower maternal weight gain and fetal weight; significant increase of blood mono- nuclear cells, platelets, and IL-6; placenta pathological examination demonstrated thrombus and chorioamnionitis |
| Chen et al. [66] | C57BL/6J mice | Diesel exhaust particles | 8.6 μg/mouse (~160 μg/m3) | Intratracheal | 3 times/week (M/W/F) beginning at 5 weeks and ending at weaning. (as mating started at 12 weeks, there was approximately a 7-week preconceptional instillation) | No impact to birth weight, however exposure significantly decreased offspring body weight from postnatal week 2 until the end of observation; decreased food intake but no alteration in brown adipose tissue |
| Chen et al. [87] | C57BL/6J mice | Concentrated ambient particles, PM2.5 | 88.66 μg/m3 | Inhalation | Preconception, pregnancy, and lactation (6 h/day, 5 days/week; no exposure took place during weekends or the day of birth) | Significantly decreased offspring birth weight, but increased body weight of adult male; adult males had increased food intake, but were sensitive to exogenous leptin |
| Wu et al. [69] | Sprague Dawley rats | Ultrafine PM, ammonium sulfate | 100 to 200 μg/m3 | Inhalation | Throughout gestation (until GD0-18) | Impacts to prenatal and postnatal organogenesis in offspring; increased stillbirths; reduced gestation length and birth weight; increased concentrations of glucose and free fatty acids in plasma; enhanced lipid accumulation in the liver; decreased endothelium-dependent relaxation of aorta |
BW birth weight, AGD anogenital distance, GD gestation day
In a rat model employing 18 days of continuous gestational exposure to ammonium sulfate particles, representative of several key PM0.1 properties observed during polluted haze events in Asia, Wu et al. [69] observed a significantly decreased gestational length, percent of offspring alive at birth, and average birth weight in the pollutant-exposed group. After weaning, offspring body weight remained markedly lower, coinciding with a significant reduction in plasma triacylglycerol concentrations. However, at the conclusion of the study (at postnatal day 105), there were no differences in body weight due to prenatal exposure in groups consuming a low- or high-fat diet. Liu et al. [89] exposed pregnant rats to PM2.5 on gestation days 10 and 18. Fetal weights were significantly decreased in the PM-exposed group. Moreover, investigators observed increased absorbed blastocysts and abnormal placental pathology following gestational PM2.5 exposure. Gorr et al. [64] also observed significantly reduced birth weights in FVB mice exposed to concentrated ambient PM2.5 throughout gestation until weaning at three weeks of age. Offspring body weights were slightly higher at 3 months of age in exposed offspring versus control. Absolute heart weights were also increased in the PM2.5 group, which correlated with significant cardiovascular dysfunction at adulthood. Manners et al. [84] observed maternal exposure to diesel exhaust PM2.5 in a mouse model employing C57Bl/6 mice resulted in offspring with significantly reduced body weights at 4 weeks of age. Other studies have confirmed early reductions in offspring birth weight following prenatal diesel exhaust particle exposure, which were sustained throughout lactation [81]. Chen et al. [87] did not observe an impact on birth weight in offspring of C57Bl/6 mice exposed to diesel exhaust PM2.5 during gestation; however, offspring body weight was found to be decreased from postnatal week two until the end of observation (20-22 weeks of age). Reduced weight gain in the PM2.5-exposed mice correlated with decreased food intake. Interestingly, reduced weight gain corresponded with increased epididymal adipose tissue mass. In contrast, in a second exposure group, where control mice were fostered by PM2.5-exposed dams, offspring body weight increased during lactation and into adulthood (without marked food intake increases). This type of study underscores how differential programming may depend upon timing of exposure.
In a separate study, Chen et al. [66] exposed C57Bl/6 mice to filtered air or concentrated ambient PM2.5 during preconception, pregnancy, and lactation or in a second exposure scheme during pregnancy and lactation only. Maternal PM2.5 exposure, including the 7-week preconception exposure, resulted in significantly decreased birth weight. Offspring exhibiting lower birth weight had a marked “catch up” growth during the lactation period, beginning after 1 week of age, making them significantly heavier than controls at time of weaning. This increase was maintained in males over the entire observation period (until 18 weeks) but only until 7 weeks of age for female offspring before converging. These data mirror the observed trend in the study by Gorr et al. [64], as well as epidemiological data supporting the “thrifty phenotype” hypothesis [36]. Chen et al. [66] also saw similar trends in the group exposed to PM2.5 during pregnancy and lactation only, yet the differences were smaller and failed to reach statistical significance. Contrary to results from Chen et al. [66], diesel exhaust PM2.5 preconception exposure at 1.0 or 3.0 DEP/m3 led to significantly lower offspring body weights at 8 weeks of age in C57Bl/6 mice [101]. Despite these differences, preconception may represent a window of vulnerability, which is often not included in human observational studies.
Respiratory and immune system effects
Rodent models employing prenatal fine and ultrafine PM exposure provide substantial evidence on offspring lung dysfunction and increased asthma susceptibility (Table 3). Features of asthma, or allergic airway disease, can be characterized by measuring airway inflammation and hyperresponsiveness following an allergen exposure [108]. Frequently, mouse models employ sensitization and challenge using the experimental allergen ovalbumin (OVA) or a human relevant allergen, house dust mite. Several models employing various exposure regimens and allergen challenges confirm prenatal PM2.5 and PM0.1 exposure leads to a characteristic asthma phenotype in offspring. Hamada et al. [78] initially demonstrated pregnant Balb/c mice exposed to aerosolized residual oil fly ash, a surrogate for ambient PM2.5, just prior to delivery significantly increased offspring OVA-induced airway hyperresponsiveness (AHR) and elevated levels of eosinophils in bronchoalveolar lavage fluid (BALF) driven by a skewed Th2 response. A primary feature of asthma involves a heightened inflammatory cell influx, largely composed of eosinophils or neutrophils driven via increased Th2 CD4+ T-cell signaling through interleukins (IL) 4 and 5 and/or Th17 signaling via IL-17. IL-4 also drives allergen-specific immunoglobin (Ig) production. Additionally, IL-13 elicits effects related to airway hyperresponsiveness (i.e., bronchoconstriction). Hamada and colleagues further highlight histopathology showing marked pulmonary inflammation and increased allergen-specific IgE and IgG levels. Fedulov et al. [83] subsequently showed pregnant Balb/c mice exhibit an acute inflammatory response to both diesel exhaust PM0.1 and more “inert” particles, titanium dioxide (TiO2), in contrast to non-pregnant females. Offspring born to mothers exposed to either type of particle went on to develop AHR and display airway inflammation following OVA challenge. These data underscore the state of pregnancy itself affects the host-response to particle exposure and confirm particles increase asthma susceptibly in offspring. In another model of prenatal exposure to diesel exhaust (DE), Corson et al. [82] co-exposed pregnant Balb/c mice to Aspergillus fumigatus allergen and DE. Offspring were challenged with this allergen at 9-10 weeks of age. Interestingly, offspring in the prenatal DE + allergen exposure group had decreased IgE production and dampened airway eosinophilia indicating potential protection against allergen-induced inflammation. These results emphasize the influence of co-exposures during gestation on asthma risk. Additionally, Sharkhuu et al. [102] demonstrated prenatal DE exposure altered some baseline inflammatory indices in the lung, which varied based on sex, but changes in response to OVA-induced inflammation were not significant in exposed mice. Manners et al. [84] combined several of the dosing strategies from previously described models to investigate the mechanisms underlying prenatal PM2.5 exposure and offspring asthma risk. Researchers showed repeated exposure to DEPs during gestation led to significant OVA-enhanced inflammation and AHR in offspring. Moreover, asthma susceptibility was associated with expression of genes regulated through oxidative stress and aryl hydrocarbon receptor (AhR) pathways. Diesel exhaust PM contains a mixture of polycyclic aromatic hydrocarbons (PAHs), known to upregulate AhR-related genes. The role of AhR in the immune response, including the production of Th2 and Th17, continues to evolve [109].
Table 3.
Summary of respiratory and immune effects from in vivo models (17 studies)
| Reference | Animal model | PM source | Dose | Route | Duration | Offspring effects |
|---|---|---|---|---|---|---|
| Hamada et al. [78] | Balb/c mice | Residual oily fish ash | 50 mg/mL | Inhalation | 30 min for 5, 3, or 1 days prior to delivery | Increased airway hyperresponsiveness and elevated levels of eosinophils in BALF; histopathology showed inflammation in lungs and increased IgE and IgG1 antigen-specific antibodies; Th2-skewed immune response |
| Fedulov et al. [78] | Balb/c mice | Diesel exhaust | 50 μg/mouse (DEP, CB, or TiO2) | Intranasal | Single nasal insufflation on GD14 | Airway hyperresponsiveness and airway inflammation, suggesting increased susceptibility to asthma |
| Mauad et al. [53] | Balb/c mice | PM2.5 | 16.8 +/− 8.3 μg/m3 | Inhalation | 4 months (24 h/day) | Decreased surface to volume ratio; reduced inspiratory and expiratory volumes |
| Corson et al. [82] | Balb/c mice | Diesel exhaust particles | Average particle concentration 1.09 mg/m3. | Inhalation | 5 times 4 days apart (before mating), then 2 times after mating | Decrease in IgE production; decrease in pulmonary eosinophils |
| Sharkhuu et al. [102] | Balb/c mice | Diesel exhaust | 0, 0.8, 3.1 mg/μL | Inhalation | 10 consecutive days (4 h/day) | Number of neutrophils in BALF and splenic T cells expressing different surface markers were differentially affected by DE concentrations and sex; female offspring exposed to DE prenatally exhibited higher protein levels in the respiratory tract compared to males |
| Reiprich et al. [103] | Balb/c mice | Diesel exhaust particles | 20 μg/mouse | Inhalation | GD7 to delivery (10 min 3 times/week) | Increased airway hyperresponsiveness, eosinophilic infiltration, and antigen-specific IgE production; pretreatment with N-acetylcysteine reversed asthmatic phenotype in pollutant-exposed offspring |
| Thevenot et al. [72] | C57BL/6J mice or Brown-Norway rats | EPFR (DCB-230) in combustion-generated PM (0.2 μm) | 200 μg/m3 | Inhalation | 7 days (30 min/day) neonatal exposure only | Increased pulmonary pathologies and development of asthma; increased airway hyperresponsiveness |
| Wang et al. [104] | C57BL/6J mice | CB-NP (unknown diameter) + MCP-230 | 50 μg/kg BW | Oropharyngeal | GD10 and GD17 (once/day) | MCP-230 exposed dams, offspring have decreased production of T helper and Tregs; Th1 and Th17 cells remained |
| Manners et al. [84] | C57BL/6J mice | Diesel exhaust particles | 50 μg/mouse | Intranasal | GD3, 6, 9, 12, 15, 18 (once/day) | Increase airway inflammation and hyperresponsiveness; increase OVA-specific IgE |
| Saravia et al. [73] | C57BL/6J mice | CDPM | 200 μg/m3 | Inhalation | 5 days (30 min/day) neonatal exposure only | CDPM+HDM challenged mice exhibited no noticeable asthma phenotype, AHR, Th2 inflammation, eosinophilia, HDM Ig-specific; CDPM induce immunosuppressive environment; CDPM suppression of Th2 response |
| Lee et al. [74] | C57BL/6J mice (neonates < 7-day age) | CDPM (0.2 μm); EPFR (DCB-50 and DCB-230) | 200 μg/m3 | Inhalation | 5 days (30 min/day) | Enhanced morbidity and decreased survival; increased oxidative stress; increased pulmonary Tregs during influenza |
| El Sayed et al. [105] | ICR mice | CB-NP (14 nm diameter) | 95 μg/kg BW | Intranasal | GD9 and GD15 (once/day) | Increased total thymocytes and immunophenotypes; increase total lymphocytes in male offspring at PND5 exposed to CBNP; upregulation of mRNA expression of genes involved with induction of peripheral tolerance |
| Paul et al. [106] | C57BL/6J mice | TiO2, CeO2, Ag nanoparticles (10 nm diameter) | 10 μL of NPs at 10 mg/mL | Intratracheal | GD2.5, GD9.5, and GD16.5 | Offspring lung development was stunted regardless of nanoparticle exposure |
| Jaligama et al. [76] | C57BL/6J mice | CDPM (0.2 μm); EPFR (DCB-230) | 200 μg/m3 | Inhalation | 7 days (30 min/day) neonatal exposure only | Increase in Tregs and IL-10; EPFR+ IL-10−/− neonates exhibited reduced morbidity, viral load, and adaptive T cell response after influenza infection |
| de Barros et al. [107] | Balb/c mice | PM2.5 | 600 μg/m3 | Inhalation | GD5.5-GD18.5 (1 h/day) | Increased lung elastance and decreased alveolar number in PND40 offspring; lung volume and BALF were not affected; transcriptomic analysis indicated DNA damage, inflammation, and cell proliferation regulation in PND40 exposed offspring |
| Tang et al. [94] | Sprague Dawley rats | PM2.5 | 0.1, 0.5, 2.5, 7.5 mg/kg | Intraperitoneal | GD0-GD18 (once every 3 days) | Ground glass opacity and high-density volumes in lungs of exposed neonates; increased pulmonary inflammation in lungs |
| Rychlik et al. [68] | C57BL/6J and Balb/c mice | Ultrafine PM | 100 μg/m3 | Inhalation | GD0-GD18 (6 h/day) | Reduced inflammatory response to HDM; lower WBC counts; less peribronchiolar inflammation; C57Bl/6J offspring exposed to UFPs had increased Treg response and decreased Th2/Th17 response |
BALF bronchoalveolar lavage fluid, DE diesel exhaust, HDM house dust mite, UFPs ultrafine particles, GD gestational day, CDPM combustion-derived PM, EPFR environmentally persistent free radicals
Asthma is a complex chronic disease with multiple elements involved in its etiology, including genetic predisposition and a variety of environmental factors. Maternal exposure to microbial-rich environments is suggested to play a protective role against childhood asthma and allergy development [110]. Using an innovative approach, Reiprich et al. [103] tested the ability of endotoxin (lipopolysaccharide, LPS), representative of microbial exposure, to protect against offspring asthma development. LPS protected against OVA-induced pulmonary inflammation and AHR; however, in offspring prenatally exposed to DEPs, LPS failed to confer protection. Investigators showed the protection was dependent on the epigenetic regulation of IFNγ expression. Maternal supplementation with the antioxidant N-acetylcysteine reversed these effects suggesting that maternal dietary supplementation may serve as a preventive intervention to combat DEP-induced oxidative stress and downstream consequences in offspring.
Structural alterations in lung development and related functional changes have also been intensely investigated in rodent models of prenatal fine and ultrafine PM exposure. Mauad et al. [53] revealed offspring exposed pre- and postnatally to heavy traffic in São Paulo presented smaller surface to volume ratios and decreased inspiratory and expiratory volumes. Subsequent research by this group substantiates these structural and functional defects [107]. While glandular and saccular structures of fetal lungs were not substantially altered following gestational exposure to concentrated urban PM2.5 and PM0.1 from São Paulo, offspring showed significantly lower alveolar number and higher lung elastance at postnatal day 40. Genes related to DNA damage, cell proliferation, and inflammation were differentially expressed in the fetal lung suggesting a complex interplay of pathways influencing long-term lung alterations. Research on prenatal PM0.1 exposure mirrors work related to manufactured nanoparticles. Paul et al. [106] showed gestational exposure to titanium dioxide (TiO2), cerium oxide (CeO2), and silver nanoparticles-induced stereotyped impairment of lung development (decreased radial alveolar count/alveolar surface) with lasting effects in adult mice. These effects were independent of the chemical nature of the nanoparticles indicating particle size played a primary role.
In a rat model, repeated gestational PM2.5 exposure resulted in significant changes in offspring lung structure and function, including increased lung consolidation, airway inflammation, and decreased lung volume and compliance [94]. Additionally, in PM2.5-exposed offspring, investigators observed interstitial proliferation, significant oxidative stress in lungs, and upregulation of epithelial-mesenchymal transition (EMT). EMT is a process where fully differentiated epithelial cells undergo transition to a mesenchymal phenotype, thus losing typical epithelial markers like E-cadherin. Transforming growth factor-beta (TGF-β), a key mediator of EMT, can be influenced by oxidative stress/reactive oxygen species (ROS), suggesting the link between PM2.5 and aberrant ROS signaling underlying the process of EMT [111]. This phenotype was also characterized in a mouse model of neonatal exposure to radical-containing ultrafine particles [72]. Investigators proposed EMT in neonatal mouse lungs following acute exposure to ultrafine particles may underlie epidemiological evidence supporting PM exposure and increased risk of asthma. Successive research from this group supports the role of early life PM0.1 exposure and increased risk of both asthma and respiratory infection risk. Saravia et al. [73] demonstrated an early immunosuppressive phenotype in mice exposed to PM0.1 and challenged with house dust mite (HDM). Herein, the PM-HDM group failed to develop the typical asthma-like phenotype; however, offspring developed an allergic phenotype upon re-challenge later in life. The “switching” observed in this study indicates the crucial importance of the timing of exposure, as well as when pulmonary assessment is conducted. Moreover, the initial immune suppression is relevant to respiratory infection risk, which investigators went on to show neonates exposed to PM0.1 early in life were more susceptible to severe influenza infection [74]. Likewise, in a mouse model of prenatal exposure to PM0.1, Rychlik et al. [68] demonstrated a dampened response to HDM challenge in offspring from the exposed group. The role of altered adaptive immune response appears to underlie the muted response. Circulating IL-10 was significantly upregulated in offspring exposed to PM0.1, suggesting increased regulatory T cell (Tregs) expression and suppressed Th2/Th17 response. Jaligama et al. confirmed the role of IL-10 and Tregs in suppressing the adaptive response following early life PM0.1 exposure [76]. Depletion of Tregs reduced morbidity and conferred enhanced protection against influenza virus.
El-Sayed et al. [105] also demonstrated gestational exposure to carbon black nanoparticles induced dysregulation of lymphocyte populations in offspring, indicating neonatal peripheral tolerance. These effects could be predictive of allergic or inflammatory responses in childhood. Importantly, the magnitude of alteration depended on the stage of gestation fetuses were exposed, highlighting the importance in the timing and duration of exposure. In support of the hypothesis that prenatal PM alters immune development and predisposes offspring toward asthma, Wang et al. [104] showed gestational exposure to combustion-derived PM0.1 inhibited offspring pulmonary T cell development, with suppression of Th1, Th2, Th17, and Tregs at 6 days of age. Pulmonary Th1 cells remained suppressed up to 6 weeks, leading to enhancement of postnatal allergic responses to OVA evidenced by increased AHR, eosinophilia, and pulmonary Th2 responses. Overall, the bulk of the non-human evidence validates prenatal PM2.5 and PM0.1 exposure alter offspring lung and immune system development, signifying risk for acute (respiratory infection) and chronic (asthma) pulmonary health outcomes. Since variations in fetal development of lung and immune system vary between humans and rodents, it is sometimes difficult to interpret the translational relevance in regards to trimester-specific effects. Nonetheless, combined evidence from human and non-human studies support reducing exposure may prevent the tremendous burden of respiratory morbidity in infants and children.
Cognitive effects
The neurodevelopmental effects of prenatal PM exposure are well-documented in numerous epidemiological studies. Likewise, in the past 10 years, the number of animal studies has expanded considerably verifying the adverse cognitive effects and underlying neurotoxic mechanisms (Table 4). In numerous models, prenatal exposure to fine and ultrafine PM leads to offspring cognitive and behavioral impairment, often in a sex-specific manner bias toward effects on male offspring. Neurodevelopmental disorders, including autism spectrum disorder (ASD), have an increased prevalence in males. Boys are approximately three times more likely to be diagnosed than girls [119]. Findings from laboratory studies highlight how PM-induced neurological damage, including structural and functional changes, are region, sex, and timing-dependent.
Table 4.
Summary of cognitive effects from in vivo models (19 studies)
| Reference | Animal Model | PM Source | Dose | Route | Duration | Offspring Effects |
|---|---|---|---|---|---|---|
| Suzuki et al. [112] | ICR mice | Diesel exhaust | 171 μg/m3 | Inhalation | GD2-GD16 (8 h/day) | Decreased spontaneous locomotor activity; neurotransmitters (dopamine and noradrenaline) and metabolites were increased in the prefrontal cortex; decreased SLA due to facilitated release of dopamine in the PFC |
| Allen et al. [55] | C57BL/6J mice | Ultrafine PM, concentrated ambient particles | ∼40,000-496,000 particles/cm3 | Inhalation | PND4–7 and PND10–13 (with and without adult exposure over PND56–60) | Increased FR response rates and FR resets, as well as enhanced bias for immediate reward. |
| Davis et al. [79] | C57BL/6J mice | nPM; < 200 nm | 350 μg/m3 | Inhalation | 5 h/day 3 days/week for 10 weeks (females exposed 7 weeks before conception to 2 days before birth) | Impaired cerebral cortex neuron development in vitro; increased depression-like phenotype in tail-suspension test (male); additional nPM exposure promoted pyramidal neuron development |
| Allen et al. [56] | C57BL/6J mice | Ultrafine PM, concentrated ambient particles | ∼40,000 to 496,000 particles/cm3 | Inhalation | PND4–7 and PND10–13 (with and without adult exposure over PND57–59) | Impaired short-term memory on the NOR; mechanistically, cortical and hippocampal changes in amino acids raised the potential for excitotoxicity, and persistent glial activation in frontal cortex and corpus callosum of both sexes |
| Allen et al. [57] | C57BL/6J mice | Ultrafine PM, concentrated ambient particles | 96 μg/m3 | Inhalation | PND4–7 and PND10–13 (with a group of males getting additional exposure on PND270) | Ventriculomegaly in males that persisted through young adulthood; males also showed a decrease in CNS cytokines (whereas females showed an increase); males exposed on PND270, showed changes in CNS neurotransmitters and glial activation across multiple brain regions and increased hippocampal glutamate |
| Yokota et al. [97] | ICR mice | Diesel exhaust | 90 μg/m3 | Inhalation | GD2-17 (8 h/day) | Increased social isolation-induced territorial aggressive behavior; increased serum testosterone levels; increased dopamine levels in prefrontal cortex and nucleus accumbens; lower serotonin in nucleus accumbens, amygdala, and hypothalamus |
| Onoda et al. [113] | ICR mice | CBNP | 2.9, 15, 73 μg/mL | Intranasal | GD5 and GD9 (once/day) | Increase in GFAP in cerebral cortex; increase aquaporin-4 in brain parenchyma; increased expression of Flt1, Sox17, Tgfa, Cyr61 |
| Cory-Slechta et al. [59] | C57BL/6J mice | Ultrafine PM, concentrated ambient particles | 45 μg/m3 | Inhalation | PND4–7 and PND10–13 (4 h/day) | Male-specific alterations in learning and memory functions, while females showed alterations in motivational behaviors but not final performance |
| Klocke et al. [61] | B6C3F1 mice | Ultrafine PM, concentrated ambient particles | 92.69 μg/m3 | Inhalation | GD0.5-16.5 (6 h/day) | Ventriculomegaly; increased corpus callosum area and reduced hippocampal area in both sexes; CC hypermyleination, microglial activation, and reduced total CC microglia in both sexes; increased iron deposition in CC of female offspring |
| Allen et al. [58] | C57BL/6J mice | Ultrafine PM, concentrated ambient particles | 96 μg/m3 | Inhalation | PND 4-7 and PND 10-13 (4 h/day for 4 days/week) | Induced inflammation/microglial activation; reduced size of corpus callosum; hypomyelination; aberrant white matter development; increased glutamate; increased repetitive and impulsive behavior |
| Kulas et al. [114] | FVB mice | PM2.5 | 46.7 μg/m3 | Inhalation | Gestation (6 h/day 5 days/week) | Deficits in spatial memory; long-term inflammation and neurodegeneration through increased levels of COX2; changes in levels of synaptophysin and Arg1 proteins; changes in the concentration of cytokines in the brain and spleen |
| Klocke et al. [62] | B6C3F1 mice | Ultrafine PM, concentrated ambient particles | 92.69 μg/m3 | Inhalation | GD0.5-16.5 (6 h/day) | Elevated iron levels in the cerebellum of females; altered cerebellar gene expression; significant enrichment in inflammation and transmembrane transport processes |
| Kloche et al. [115] | B6C3F1 mice | Ultrafine PM, concentrated ambient particles | 92.69 μg/m3 | Inhalation | GD0.5-16.5 (6 h/day) | Premature maturational shift in number and proportion of total cells and hypermethylation in both sexes; ventriculomegaly in females (possible amelioration of ventriculomegaly in males); alterations in cycling Ki67+/Olig2+ cell number and proportion of total cells in the female corpus collosum; total CC cellularity was slightly elevated in males |
| Woodward et al. [116] | Sprague Dawley rats | TRAP | 240 μg/m3 | Inhalation | GD2, through gestation, until 25 weeks of age (5 h/day, 3 days/week) | At 5 month males had 70% fewer newly generated neurons in the dentate gyrus (DG) of the hippocampus; microglia were activated in DG/CA1 subfields (35% more Iba1); altered blood-brain-barrier, with a 75% decrease of the tight junction protein ZO-1 in the CA1 layer, and twofold more iron deposits, a marker of microhemorrhages; impaired contextual memory, reduced food- seeking behavior, and increased depressive behaviors |
| Sobolewski et al. [60] | C57BL/6J mice | Ultrafine PM, concentrated ambient particles | 45 μg/m3 | Inhalation | PND4–7 and PND10–13 (4 h/day) | Decreased serum T concentrations; social nose-to-nose sniff rates with novel males in adulthood; adult T serum concentrations were positively correlated with nose to nose sniff rates |
| Chang et al. [98] | C57BL/6J mice | Diesel exhaust | 250-500 μg/m3 | Inhalation | GD0 to PND21 | Deficits in all three of the hallmark categories of ASD behavior: reduced social interaction, increased repetitive behavior; reduced or altered communication |
| Church et al. [63] | B6C3 mice | Ultrafine PM, concentrated ambient particles | 135.8 μg/m3 | Inhalation | Gestation followed by additional exposures to both dams and their litters from PND2-10 | Significantly decreased sociability in both sexes; however, reductions in reciprocal social interaction/increased grooming behavior were only present in males |
| Cui et al. [117] | ICR mice | Diesel exhaust particles | 0.4 mg/m3 | Inhalation | GD1.5-GD15.5 (6 h/day) | Longer total distance and time in open field test; increased expression of dopamine receptors (Drd1a, Drd2, Drd3, Drd4, Drd5) |
| Morris-Schaffer et al. [118] | C57BL/6J mice | Ultrafine elemental carbon | 50 μg/m3 | Inhalation | PND4–7 and 10–13 (4 h/day) | No significant difference in anogenital distance, BW, or CNS pathological; no changes in novel object recognition; elevated plus maze performance, FI, or DRL schedule-controlled behavior |
GD gestational day, PND postnatal day, FR fixed ratio, NOR novel object recognition, CC corpus callosum
Findings from Cory-Slechta and colleagues demonstrate early postnatal exposure to concentrated ambient ultrafine PM altered behavioral responses related to impulsivity, with exposed mice showing a preference for immediate reward [55]. This window of exposure is critical to central nervous system development and equivalent to human 3rd trimester brain development [51]. Following exposure, both sexes showed impaired learning and short-term memory outcomes and persistent glial activation in the frontal cortex and corpus callosum [56]. Exaggerated microglial activation and accompanying inflammation can have detrimental effects on neurodevelopment [120]. Increased expression of the glial fibrillary acidic protein (GFAP), a marker of activated astrocytes, has consistently been correlated with developmental PM2.5 and PM0.1 exposure [113, 114]. Additional phenotypes related to impulsivity measured using fixed interval (FI) schedule-controlled performance tests reveal varied results based on sex and timing of exposure [56]. Changes in dopamine and glutamate systems known to mediate FI performance coincided with phenotypic changes. Findings related to altered locomotor effects also signify the role of dopamine pathway activation, as well as glycine signaling inhibition [112, 117]. Supplementation with a dopamine receptor antagonist and glycine receptor agonist attenuated adverse effects [117].
Overall, sex-specific behavioral changes are consistently observed. Male-specific learning/memory-related deficits have occurred even at low level neonatal ultrafine PM exposure, whereas female neonates have displayed altered motivational behaviors but not changes in overall performance [59]. Allen et al. [57] verified early postnatal PM0.1 exposure led to ventriculomegaly, i.e., lateral ventricle dilation, preferentially in male mice that persisted through young adulthood. In complementary work, Klocke et al. [61] investigated the impact of in utero ultrafine PM exposure on offspring neurodevelopment. Exposure to concentrated ambient ultrafine particles from gestation days 0.5-16.5 also resulted in ventriculomegaly, increased corpus callosum (CC) area and reduced hippocampal area in male and female offspring. Both sexes demonstrated increased microglial activation and reduced total CC microglia number and CC hypermyelination. CC iron (Fe) deposition was increased in female offspring. Altered Fe deposition can underlie oxidative stress and indicate pathologic conditions [121]. Subsequently, Klocke et al. [62] observed a similar increase in cerebellum Fe concentrations in female offspring exposed prenatally. Iron, as well as aluminum and silicon inclusions, also presented in the CC with ultrastructural myelin sheath damage. Long-term myelin status assessed at a stage of early brain maturity revealed persistent hypermyelination in PM-exposed offspring of both sexes [115]. Findings from Woodward et al. [116] confirm glial activation and accumulation of Fe deposits. In their model, rats were exposed ultrafine PM < 200 nm diameter collected near a Los Angeles freeway throughout gestation into adulthood. Investigators only followed male offspring, and at 5 months of age observed microglia activation and increased Fe deposition. Exposed animals showed behavioral deficits, including increased depressive behavior.
Depressive behavior, aggression, and behavioral features of autism spectrum disorder (ASD) are documented across several rodent models. Davis et al. [79] exposed mice to PM0.1 collected from an urban freeway (as detailed above) prior to mating and throughout gestation. Male offspring displayed increased depression-like responses. Additionally, Yokota et al. [97] observed male offspring exposed to low level DE during the prenatal period had significantly greater social isolation-induced territorial aggressive behavior in comparison to control mice. This behavior correlated with higher serum testosterone levels in exposed males. Likewise, dopamine levels were higher in the prefrontal cortex and nucleus accumbens, a functional part of the reward system, whereas serotonin levels were lower in the nucleus accumbens, amygdala, and hypothalamus in socially isolated DE-exposed mice. Sobolewski et al. [60] corroborated some of these male-biased hormonal and neurochemical changes in a model of early postnatal ultrafine PM exposure. Male offspring had lower serum testosterone levels following exposure and showed male social novelty preference, suggesting social communication deficits. Chang et al. [98] demonstrated mice exposed to diesel exhaust throughout gestation exhibited deficits in three of categories characteristic of ASD-related behavior, including reduced social interaction, increased repetitive behavior, and reduced/altered communication. Church et al. [63] corroborated these effects linking early life PM2.5 exposure with an ASD phenotype. Mice exposed to fine PM during gestation and the early neonatal period displayed reduced sociability in both sexes and increased repetitive deficits in male offspring. In a model of early postnatal ultrafine PM exposure, Allen et al. [58] observed a pattern of developmental neurotoxicity aligned with the mechanistic underpinnings of ASD. PM-exposed offspring displayed neuroinflammation, microglial activation, reduced CC area and associated hypomyelination, aberrant white matter development, ventriculomegaly, elevated glutamate and excitatory/inhibitory imbalance, increased amygdala astrocytic activation, and repetitive and impulsive behaviors, many endpoints male-biased. Collectively, these findings along with structural and functional changes highlighted above emphasize the vulnerability of the developing brain to early life fine and ultrafine PM exposure. Studies teasing apart the critical components of PM responsible for adverse cognitive effects highlight the role of reactive metals, as well as PAHs [122], in eliciting pathology and behavioral dysfunction, versus the elemental carbon fraction alone [118].
Cardiometabolic effects
Accumulating evidence supports an impact of prenatal fine and ultrafine PM exposure on diverse metabolic diseases in offspring, such as diabetes and obesity, as well as adverse effects on the cardiovascular system. Cardiac dysfunction precedes overt heart failure, and classic risk factors include high blood pressure (hypertension), heart attack (myocardial infarction), enlargement of the heart (cardiomyopathy), and diabetes. While well-established in epidemiologic studies of adults and elderly exposed to fine PM [123], evidence of cardiac dysfunction following developmental exposure to PM2.5 and PM0.1 is only now emerging [42] (Table 5). The fetal origins of coronary disease are well-recognized by the “Barker hypothesis,” wherein fetal undernutrition manifesting initially as intrauterine growth retardation/low birth weight precedes hypertension, coronary heart disease, and non-insulin-dependent diabetes in adulthood [126]. Since 2013, several rodent models published support the findings that prenatal PM2.5 and PM0.1 exposures cause offspring cardiac dysfunction and heart disease risk later in life.
Table 5.
Summary of cardiometabolic effects from in vivo models (13 studies)
| Reference | Animal model | PM source | Dose | Route | Duration | Offspring effects |
|---|---|---|---|---|---|---|
| Weldy et al. [99] | C57BL/6J mice | Diesel exhaust PM2.5 | 300 μg/m3 | Inhalation | 3 weeks prior to mating; duration same through gestation (6 h/day, 5 days/week) | Increased susceptibility to cardiac hypertrophy, systolic failure, myocardial fibrosis, pulmonary congestion |
| Gorr et al. [64] | FVB mice | PM2.5 | 51.69 μg/m3 | Inhalation | GD0-PND21 (6 h/day, 7 days/week) | Reduced birth weight; reduced left ventricular fractional shortening; reduced ejection fraction; increased end-systolic volume; reduced dP/dt maximum; reduced peak shortening; slower calcium reuptake; reduced response to B-adrenergic stimulation; increased collagen deposition |
| Wei et al. [54] | Sprague Dawley rats | Ambient air | 73.5 +/− 61.3 μg/m3 | Inhalation | 19 days (24 h/day) | Increased body weight; peribronchiolar and perivascular inflammation; increased systemic and oxidative stress; dyslipidemia; increased inflammatory status of epididymal fat |
| Stephenson et al. [75] | C57BL/6J mice | Ultrafine PM MCP-230 | 50 μL | Oropharyngeal | GD10 and GD 17 (once/day) | Reduced skeletal muscle mtDNA copy number; lower mRNA levels of electron transport genes; reduced citrate synthase activity; upregulation of genes in reducing oxidative stress in muscle |
| Tanwar et al. [65] | FVB mice | PM2.5 | 73.61 μg/m3 | Inhalation | Gestation (6 h/day, 7 days/week) | Reduced fractional shortening; increased left ventricular end-systolic and end-diastolic diameters; reduced ventricular posterior wall thickness; end-systolic elastance; contractile reverse; increased collagen deposition; increases in cardiac IL-6, IL-1B, collagen-1, MMP9, and MMP13 gene expression |
| Goodson et al. [124] | C57BL/6J mice | Diesel exhaust | 300 μg/m3 | Inhalation | Gestation (6 h/day, 5 days/week) | Dysregulated gene expression of Mir133a-2, Ptprf, Pamr1; promoter of Mir133a-2 differentially methylated |
| Harrigan et al. [100] | C57Bl/6 (ApoE−/−) mice | Diesel exhaust particles PM2.5 | 250-300 μg/m3 | Inhalation | Gestation (6 h/day, 5 days/week) | Smaller litter and increased postnatal mortality; no significant differences in plasma lipids or lipoprotein profiles, expression of antioxidant genes or markers of oxidative stress between groups; no sig. differences in atherosclerotic lesion area |
| Chen et al. [86] | C57BL/6J mice | Diesel exhaust particles PM2.5 | 160 μg/m3 | Intratracheal | 7-week preconception period, whole gestation, and lactational periods (3 times/week) | Impaired adult male offspring glucose tolerance; no influence in insulin sensitivity for adult male offspring; decreased insulin secretion; decrease in pancreatic islet and Beta cell sizes |
| Ye et al. [125] | Sprague Dawley rats | PM2.5 | 1 mg/kg BW | Oropharyngeal | GD8, 10, 12 (once/day) | Increased blood pressure; impaired sodium excretion; reduced D1R-mediated natriuresis and diuresis; increased D1R and GRK4 expression |
| Wu et al. [69] | Sprague Dawley rats | Ultrafine PM (10-20 nm diameter; ammonium sulfate particles) | 150 μg/m3 | Inhalation | GD0-GD18 | Increased stillbirths; reduced gestation length and birth weight; increased concentrations of glucose and free fatty acids in plasma; increased lipid accumulation in liver; decreased endothelium-dependent relaxation of aorta |
| Morales-Rubio et al. [92] | C57BL/6J mice | Ultrafine PM (< 60 nm) | 50 μL UFP followed by 200 μL air (400 μg/kg BW) | Intratracheal | GD6.5, 8.5, 10.5, 12.5, 14.5, 16.5 (once/day) | Increased embryo reabsorption; decreases in uterus, placental, and fetal weights; HSD11B2 hypermethylation and protein downregulation; increases in PAH enzymes; activation of AT1R and ACE in PND50 resulting in increased blood pressure |
| Woodward et al. [80] | C57BL/6J mice | Ultrafine PM (< 100 nm) | 343 μg/m3 | Inhalation | Gestation (5 h/day, 3 days/week) | Increased food intake, body weight, fat mass, adiposity, glucose intolerance; dysregulation of neuropeptides in hypothalamus; decreased expression of insulin receptor signaling genes in adipose |
| Xie et al. [90] | C57BL/6J mice | PM2.5 | 0.3 mg/kg/day | Oral | GD0-GD18 (once/2 days) | Reduced BW in adult males after 6 weeks; reduced adipocytesize in eWAT compared to BAT; decreased expression of ACC1, ACSL1, PPAR-a in eWAT; decreased expression of TNF-a, IL-1B, IL-6; reduction of LPC, PC, PE, SM, Cer. |
GD gestational day, BW body weight
Weldy et al. [99] exposed C57Bl/6 mice to filtered air or diesel exhaust (DE) prior to mating for 3 weeks, during gestation, and until offspring were 8 weeks of age. At 12 weeks of age, male offspring underwent a transverse aortic constriction (TAC) surgery to induce pressure overload. Exposed mice showed increased risk of cardiac hypertrophy, systolic failure, myocardial fibrosis, and pulmonary congestion following TAC surgery, indicative of risk for heart failure. A study by Gorr et al. [64], in which investigators exposed FVB mice to PM2.5 during pregnancy and continuing until time of weaning concluded that early life exposure to fine PM leads to cardiac dysfunction. At adulthood, exposed mice showed reduced left ventricular fractional shortening, with greater left ventricular end-systolic diameter. Pressure-volume loops revealed alterations in several key parameters associated with dysfunction. Histological analyses highlighted increased cardiac collagen deposition, a precursor to fibrosis. Tanwar et al. [65] further clarified the mechanisms of heart failure susceptibility following in utero exposure to PM2.5. Investigators demonstrated several adverse phenotypes in exposed offspring evaluated at 12 weeks, including reduced fractional shortening, increased left ventricular end-systolic and end-diastolic diameters, reduced left ventricular posterior wall thickness, end-systolic elastance, contractile reserve, frequency-dependent acceleration of relaxation, and blunted contractile response to β-adrenergic stimulation. Moreover, histological assessment showed increase collagen deposition in exposed offspring, confirming findings from Gorr et al. [64, 65]. Acute inflammatory markers, alterations in Ca2+ handling proteins, and changes in protein expression of DNA methyltransferases were also marked in the exposed group, suggesting the role of epigenetic changes in priming heart disease risk. Goodson et al. [124] subsequently demonstrated in utero exposure to DE altered DNA methylation in cultured neonate cardiomyocytes.
Additional studies have investigated the impact of developmental fine and ultrafine PM exposure on blood pressure and vessel vasodilatory response. Wu et al. [69] showed adult Sprague-Dawley rats exposed to ultrafine PM throughout gestation had impaired relaxation of the nitric oxide (NO)-mediated vasodilatory response of their aortas. A hallmark of cardiovascular dysfunction is a reduced bioavailability of NO, a major vasodilator, from endothelial cells [127]. PM-driven reactive oxygen species (ROS) can react with NO to yield reactive peroxy species (e.g., peroxynitrite), and this reaction leads to both a NO deficiency and a depletion of reduced glutathione (the major antioxidant in cells), constituting a vicious cycle [128]. Morales-Rubio et al. [92] further showed in utero exposure to ultrafine PM induced placental stress in mice, via intrauterine oxidative damage and inflammation, stimulating programming and activation of the angiotensin II receptor type 1 (AT1R) and angiotensin I-converting enzyme (ACE) in offspring lung. These changes correlated with increased blood pressure in male offspring around 7 weeks of age. Ye et al. [125] investigated the role of the regulation of renal sodium transport in increased blood pressure following in utero exposure to fine PM. In this study, Sprague-Dawley rats were repeatedly exposed to PM2.5 during pregnancy; exposed offspring increased offspring blood pressure and decreased renal G protein-coupled receptor kinase type 4 (GRK4) expression, a receptor that plays an important role in sodium transport. Furthermore, exposed offspring had impaired renal dopamine D1 receptor-mediated sodium excretion. Strikingly, these abnormalities were normalized by administration of the antioxidant tempol, suggesting antioxidants may serve as potential therapeutics for PM-mediated hypertension.
While these studies signify the potential for developmental PM2.5 and PM0.1 exposure to influence hypertension, a major risk factor for heart disease, another risk factor, atherosclerosis, was not shown to be a consequence of prenatal exposure in a recent model. Harrigan et al. [100] exposed pregnant apolipoprotein E-deficient mice (Apoe−/−) to DE throughout gestation. The Apoe−/− mouse model is useful for studying cardiovascular disease since mice have poor lipoprotein clearance, which supports cholesterol accumulation in blood, thereby promoting the development of atherosclerotic plaques [129]. While there was higher postnatal mortality in the DE-exposed mice, there were no significant differences in plasma lipids or lipoprotein profiles, expression of antioxidant genes or markers of oxidative stress between treatment groups at 16 weeks of age. DE-exposed offspring did present a higher frequency of atherosclerotic lesions, but overall, there were no significant difference in average atherosclerotic lesion area in the aortic sinus or innominate arteries. Likewise, Wu et al. [69] did not observe differences in concentrations of non-esterified fatty acids in plasma of male and female rats at 3 weeks of age following prenatal exposure to ultrafine PM or filtered air. Paradoxically, investigators measured significant decreases in plasma triacylglycerol concentrations in PM0.1-exposed offspring (both male and female). Wei et al. [54] chronically exposed rats to either filtered or unfiltered polluted Beijing air throughout gestation to 8 weeks of age. Blood markers showed exposure to unfiltered air resulted in a worsened lipid profile, reduced GLP-1 levels, an incretin hormone that reinforces glucose-dependent secretion of insulin, and reduced antioxidant capacity parallel with measures of increased oxidative stress. Additionally, epididymal fat mass was greater in the male offspring exposed to polluted air. Overall, these findings based on three studies (in different models with distinct exposure durations and varying pollutants) necessitate additional mechanistic studies to determine the role of developmental fine and ultrafine PM exposures on plasma lipid profiles. However, findings from Wei et al. [54] related to effects on glucose homeostasis and fat accumulation are substantiated by additional studies detailed below.
Several metabolic disease states are characterized, including diabetes phenotypes (i.e., disruption of glucose metabolism) and obesity risk. Largely, studies show prenatal PM2.5 and PM0.1 exposure can alter glucose homeostasis in offspring, in particular male offspring. Chen et al. [86] demonstrated prenatal exposure to DE PM2.5 in C57Bl/6 mice altered the morphology and function of male offspring pancreatic β cells, which are responsible for producing insulin, thus altering glucose metabolism. This deficiency correlated with decreased pancreatic islet cell and β cell sizes. Woodward et al. [80] observed a sex-specific impairment of glucose tolerance in C57Bl/6 male offspring exposed to PM0.1 during gestation through 17 weeks of age. These changes correlated with increased body weight, fat mass, adiposity, and increased food intake, representing an altered feeding behavior. Underlying these effects were significant changes in expression of metabolically relevant neuropeptides in the hypothalamus and decreased expression of insulin receptor signaling genes in adipose tissue. Male susceptibility was further confirmed in a study by Xie et al. [90], although in this model offspring exhibited sustained growth inhibitory effects, and not obesity. Following prenatal exposure to PM2.5, male mice showed reduced body weight beginning at 6 weeks of age, along with decreased epididymal adipose tissue, where there was a concurrent downregulation of genes related to fatty acid synthesis and oxidation. Lipidomic analysis revealed disruptions in sphingomyelin-ceramide signaling and glycerophospholipids remodeling. Notably, investigators did not carry out this analysis in female offspring since no changes in body weight were observed over the 5- to 15-week postnatal growth period.
These data mirror the varying growth effects observed in epidemiologic and animal studies, where different models report differing impacts on longitudinal growth in offspring. Indeed, Chen et al. [66] reported obesity in male offspring following prenatal exposure to PM2.5 in their mouse model employing concentrated ambient particle (CAP) exposure. Here, male offspring showed increased food intake, but were sensitive to exogenous leptin, a hormone involved in the regulation of energy intake and expenditure [130]. Leptin promoter methylation in adipocytes was significantly increased in PM2.5-exposed males but not females, signifying the role of altered leptin in programing male obesity. Stephenson et al. [75] reported that male mice exposed in utero to combustion-derived PM0.1 had increased weight gain in comparison to controls after placement on a high-fat diet for 12 weeks (from 10-22 weeks of age). Increased body weight was not associated with increased food intake, but correlated with lessened physical activity and energy expenditure. This corresponded with reductions in skeletal muscle mitochondrial DNA copy number, lower expression of electron transport genes, and reduced citrate synthase activity. Although the mechanisms behind these changes remain to be determined, accumulating findings that prenatal fine and ultrafine PM exposure can alter glucose metabolism later in life highlights a need for further research in this area, especially based on the current childhood obesity epidemic [30].
Biological mechanisms underlying adverse effects
The range of adverse health outcomes in children following early life fine and ultrafine PM exposure are understood to be caused through two broad mechanisms, (1) directly crossing the placenta, hence, reaching fetal circulation and/or (2) indirectly through an interplay of PM-driven maternal/placental oxidative stress, inflammation, epigenetic modifications in placental and offspring tissues, and potential endocrine disruption [131, 132].
Placental translocation of ultrafine particles
The placenta is a multifunctional organ that connects the developing fetus to the uterus through the umbilical cord. The primary function to provide oxygen and nutrients, remove waste products, and importantly, serve as a barrier between maternal and fetal tissues. Evidence from in vivo animal exposure models, ex vivo human placental models, and recent direct evidence from human placentae demonstrate transport of ultrafine particles (UFPs) across the placental barrier indicating possible direct effects on placental function and offspring health [133, 134].
In a rabbit model of diesel exhaust exposure, filtered to generate ultrafine PM (~69 nm diameter particles), Valentino et al. [135] observed transplacental transfer of particles using transmission electron microscopy (TEM) analysis. Nanoparticles were visualized in maternal blood spaces of exposed placentae, in trophoblastic cells, and within fetal vessels. Investigators also demonstrated disruption of placental function in exposed animals, which was largely due to reduced placental vascularization. Veras et al. [136] also reported functional morphologic changes in placentae, primarily on the maternal side, from mice exposed to ambient levels of air pollution in São Paulo. Exposure during gestation resulted in smaller fetal weights, reduced volumes, calibers, and surface areas of maternal blood spaces, and greater fetal capillary surfaces and diffusive conductance, which authors discussed as a fetoplacental adaption to maintain and expand oxygen and nutrient delivery. In another mouse model, Paul et al. [106] confirmed decreased placental efficiency with the presence of metallic nanoparticles in the placenta. This correlated with impaired lung development in offspring that persisted into adulthood.
Studies in human placentae have also verified placental translocation of ultrafine particles. Wick et al. [137] demonstrated the size-dependent transport of fluorescent polystyrene particles with diameters of 50, 80, and 240 nm (but not 500 nm) across human placental explants into the fetal circuit. Bové et al. [134] was the first research group to visualize particles on the fetal side of human placentae in a maternal cohort study. Black carbon (BC) particle aggregates ranged between 1.00 and 9.78 μm and consisted of various smaller particles that translocated from maternal circulation to distinct locations inside the placental tissue. Overall placental BC load positively correlated with maternal BC residential exposure averaged over pregnancy, indicating a dose-response relationship. To fully understand direct impacts of ultrafine PM on placental function and offspring health, further studies are needed that consider the toxicity associated with various PM constituents, as well as the role of metabolic activation in placental tissue.
Placental and systemic maternal oxidative stress and inflammation
Exposure to fine and ultrafine PM elicits maternal systemic and placental oxidative stress and inflammation through various mechanisms [138]. Oxidative stress is generally defined as an imbalance of pro-oxidants and antioxidants driven by an increase in free radicals, namely, reactive oxygen species (ROS). From a mechanistic standpoint, a refined definition also considers oxidative stress a disruption of redox signaling and control without the characteristic involvement of free radicals [137, 139]. PM2.5 and PM0.1 can induce typical free radical-driven macromolecular damage (i.e., protein modifications, DNA oxidation, and lipid peroxidation), as well as alter the redox states of thiol systems controlled by the thioredoxins (Trx), glutathione (GSH), and cysteine (Cys). The mechanisms of PM2.5-associated adverse perinatal outcomes have been previously reviewed [138, 140]. In this section, an update on the mechanisms and evidence in human and animal studies will be discussed.
Briefly, direct generation of ROS from transition metals adsorbed to fine and ultrafine PM, like iron, copper, chromium, and vanadium, work through interactions with superoxide or hydrogen peroxide to form reactive hydroxyl radicals that can damage macromolecules. Metals and organic constituents, like PAHs, adsorbed to PM2.5 and PM0.1 have high oxidative potential and ability to generate mitochondrial damage [141, 142]. Mitochondrial damage can lead to excess NADPH-oxidase and superoxide production/release. PAHs can also be activated to reactive metabolites via cytochrome (CYP) P450 metabolism. CYPs are transcriptionally upregulated through ligand-activation of the aryl hydrocarbon receptor (AhR). CYP1A1 is the most important xenobiotic-metabolizing enzyme expressed in the placenta, for which activation has been associated with numerous pregnancy-related complications [143]. Toxicological consequences of reactive metabolites, i.e., quinones and epoxides, act through formation of DNA and protein adducts leading to mutagenicity and toxicity. Phase II enzymes, such as glutathione s-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), and NAD(P)H-dependent quinone oxidoreductase-1 (NQO1) detoxify reactive metabolites by catalyzing conjugation reactions and the reduction of quinones to hydroquinones, respectively. Phase II enzymes are transcriptionally upregulated through nuclear factor erythroid 2-related factor (Nrf2) signaling.
Nrf2 is a redox sensitive transcription factor regarded as the master regulator of the antioxidant response [144]. Nrf2 binding to the antioxidant response element (ARE) in promoters upstream of phase II enzymes and other oxidative stress-related enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and catalase (CAT), drives transcription in response to oxidative stress. The Nrf2 antioxidant response pathway plays an important role in response to PM-induced oxidative stress. Findings from a birth cohort in Korea demonstrating increased susceptibility of lower respiratory tract infections in infants prenatally exposed to PM2.5 was significantly modified by polymorphisms in the maternal Nrf2 gene [145]. The risk was increased when investigators included prenatal exposure to environmental tobacco smoke. Additionally, disruption of Nrf2 has been shown to enhance susceptibility to allergic airway inflammatory responses induced by chronic exposure to diesel exhaust PM [146]. Supplementation with thiol antioxidants that activate Nrf2 has been shown to reduce the allergic inflammatory effects of DEPM in vivo [147], a possible preventive intervention approach discussed in the following section. Crosstalk between the AhR and Nrf2 pathways may also be important in response to PM. Shin et al. [148] demonstrated Nrf2 is activated by AhR and can also induce AhR expression. Alternatively, PM-mediated oxidative stress can induce inflammation, sometimes referred to as the tier 2 response preceding tier 3 cytotoxicity or cell death [149, 150]. In the second tier of oxidative stress, the activation of signaling cascades, like the redox-sensitive transcription factor NF-κB, leads to activation of pro-inflammatory cytokines and other immune response genes [151]. Activation of this pathway is associated with increased levels of the pro-inflammatory enzyme COX-2 and cytokines like IL-1β and TNF-α, drivers of systemic inflammation. Complex molecular mechanisms link the Nrf2 and NF-κB pathways. Lack of Nrf2 activity can exacerbate NF-κB signaling, leading to increased cytokine production; alternatively, NF-κB can modulate Nrf2 activity, having both positive and negative effects on target gene expression [152, 153].
Fine PM exposure induces maternal systemic and placental oxidative stress and inflammation, as evidenced in exposed human populations and in experimental models. Nagiah et al. [154] observed pregnant women living in highly industrialized areas of south Durban, South Africa, had increased markers reflective of oxidative stress in circulating lymphocytes as compared to women living in a less industrialized area in the north with lower pollutant levels. Levels of malondialdehyde (MDA), superoxide dismutase (SOD2), and uncoupling protein 2 (UCP2) mRNA were elevated, whereas Nrf2 and GSH expression was decreased in the higher exposure group. Similarly, Ambroz et al. [155] compared urine and blood markers reflecting oxidative DNA damage and lipid peroxidation in mothers and infants living in the Czech Republic in areas with relatively poor to good air quality. Investigators measured 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodG), a common product of DNA oxidation, and 15-F2t-isoprostane (15-F2t-IsoP), an oxidized product of arachidonic acid. Isoprostanes, including 15-F2t-IsoP, as well as 8-iso-prostaglandin F2α (8-iso-PGF2α), are routinely employed as markers of oxidative stress. In newborns from the Czech Republic, PM2.5 concentrations significantly predicted 8-oxodG excretion, and PM2.5 and benzo[a]pyrene concentrations significantly predicted 15-F2t-IsoP levels. In mothers, PM2.5 concentrations were a significant predictor of 8-oxodG levels. Using a metabolomics approach, Yan et al. [132] observed maternal oxidative stress and inflammation-related pathways, including linoleate, leukotriene, and prostaglandin, were altered in response to traffic-related air pollution exposure.
Epidemiological studies also reveal positive associations between PM2.5 exposure and plasma homocysteine levels, an established marker of cardiovascular disease risk [156]. Homocysteine, a thiol-containing amino acid, is produced by the intracellular demethylation of methionine. Normally, levels of homocysteine are low due to rapid metabolism. In a cohort study, investigators confirmed significantly elevated umbilical cord blood homocysteine levels that were on average 8.1% higher for every 5 μg/m3 increase in PM2.5 [157]. Interactions with single nucleotide polymorphisms (SNPs) in certain oxidative stress-related enzymes suggested that oxidative stress may be an underlying mechanism. Earlier findings from this cohort revealed placental nitrosative stress, measured via 3-nitrotyrosine concentrations, were significantly associated with increased PM2.5 and BC exposure during pregnancy [158]. Markers of mitochondrial damage are also documented in mothers and newborns exposed to PM2.5 during gestation. Alterations in mitochondrial DNA (mtDNA) can serve as a marker of cumulative oxidative stress based on the mitochondria’s lack of repair systems. Rosa et al. [159] observed mtDNA content from umbilical cord leukocytes was inversely correlated with PM2.5 exposure during pregnancy. These findings were confirmed by Brunst et al. [160], wherein PM2.5 exposure across pregnancy was associated with decreased mtDNA copy number in cord blood. Grevendonk et al. [161] demonstrated PM2.5 exposure during pregnancy was positively associated with markers of mitochondrial DNA damage in maternal blood.
Telomere length is another measure affected by oxidative stress, and shortening has been related to biological aging. Cord blood and placental telomere length have both been significantly inversely associated with PM2.5 exposure [162]. Cord blood leukocyte telomeres were on average 8.8% shorter and placental telomere lengths were 13.2% shorter for every 5 μg/m3 increase in PM2.5. Rosa et al. [163] reported shorter (but sometimes longer) leukocyte telomere length in association with PM2.5 during specific prenatal windows and differences based on neonatal sex. PM2.5 was more strongly associated with shortened telomere length in girls as compared to boys. Complex sex and other psycho-social stressor interactions may be important modifiers of markers of oxidative stress, like telomere length and mtDNA. For instance, findings by Brunst et al. [160] indicated the impact of maternal trauma on increased placental mtDNA copy number. In general, psychosocial stress, diet/nutrition, and other maternal exposures like smoking share similar pathways with that of particulate air pollution exposure, which could potentially exacerbate the negative effects of either insult alone [138]. Abundant evidence also indicates gestational PM2.5 exposure promotes a systemic pro-inflammatory response and stimulates placental inflammation [164]. Investigators observed PM2.5 exposure was associated with increased C-reactive protein (CRP) concentrations in early pregnancy [165]. CRP is a biomarker of systemic inflammation and is often elevated in pregnancy-related conditions [166]. Nachman et al. [167] reported a positive relationship between PM2.5 exposure during preconception and pregnancy and intrauterine inflammation. Models of gestational fine and ultrafine PM exposure confirm intrauterine oxidative damage and inflammation [83, 92].
Additionally, there is substantial evidence from animal models to support findings in exposed pregnant women and developing fetuses. Generally, the two mechanisms (PM-induced oxidative stress and inflammation) are studied concurrently. Pregnant mice exposed to DE displayed increased placental expression of immunomodulatory cytokines, including IL-2, IL-5, IL-12, and granulocyte monocyte colony stimulating factor, GM-CSF [102]. In another mouse model, DE exposure increased placental expression of multiple inflammatory cytokines, especially IL-5 and IL-6 (<tenfold) [168]. IL-4 and IL-6 levels have also been shown to increase in response to gestational PM2.5 exposure [125]. de Melo et al. [169] further reported increased IL-4 expression in the fetal portion of the placenta in rats exposed to fine PM before and during pregnancy. While most literature agrees that PM2.5 induces oxidative stress and inflammatory states, there are a few conflicting results. Biomarkers of systemic inflammation measured in peripheral blood in an ICR mouse model employing gestational PM2.5 exposure indicated increased IL-2, IL-6, IL-8, and TNFα [170]. Interestingly, there were no differences in glutathione; however, catalase decreased while heme oxygenase increased indicating PM2.5-induced oxidative stress. Pregnant rats exposed to a low dose of PM2.5 via intratracheal instillation on gestational days 10 and 18 showed increased IL-6 levels in maternal blood; however, oxidative stress appeared to be less important in this model [89]. There were no differences in GSH-Px and MDA of placenta homogenate between exposed and non-exposed groups. In another mouse model, polluted ambient air exposure from a busy street was associated with thinner umbilical cord walls and increased lipid peroxidation leading to decreased fetal weights [171]. Specifically, the structural changes in umbilical vessels were associated with greater volumes of regions immunostained for 15-F2t-IsoP. Wang et al. [104] confirmed increased 8-isoprostanes in plasma of PM0.1-exposed dams. Morales-Rubio et al. [92] verified gestational UFP exposure increased PAH-biotransforming enzymes, intrauterine inflammation and oxidative damage, displayed by increased 8-OHdG in mouse placentae. Last, Song et al. [172] revealed changes in molecular clock gene expression in pregnant rats and their offspring exposed to heavily polluted air. Investigators suggested changes in the regulation of circadian rhythms may be another important pathway for explaining the feedbacks of air pollution exposure in addition to oxidative stress and inflammation. Overall, there is considerable evidence from human and nonhuman studies supporting exposure to fine and ultrafine PM causes adverse effects via oxidative stress/redox signaling imbalance and pro-inflammatory responses.
Epigenetic alterations
While epigenetic changes in response to fine PM have been documented across lifespan [173], this section will focus on epigenetic alterations following developmental PM2.5 exposure. Epigenetics, defined as mitotically or meiotically heritable changes in gene expression without a change in DNA sequence, was first defined by Conrad Waddington in the 1940s [174–176]. Emerging evidence from multiple human cohort studies suggests that gestational fine PM exposure induces several interconnected pathological processes through certain chemical modifications to DNA, mainly methylation, and non-coding RNAs, forming a complex regulatory network that modulates gene expression, contributing to systemic oxidative stress and altered molecular cell signaling pathways. The exact mechanisms regarding PM-induced epigenetic alterations and resulting impact on offspring have been vague and awaits further breakthroughs on many fronts.
DNA methylation is one of the most widely investigated epigenetic modifications and is recognized as one of the mechanisms linking prenatal PM2.5 exposure and adverse offspring health outcomes [177]. Studies support gestational PM2.5 exposure results in altered DNA methylation, including global hypomethylation in placental tissue [178], as well as gene specific alterations in methylation. It is recognized that epigenetic stability is proportional related to DNA methylation, as global hypomethylation of DNA may lead to genomic instability [179, 180]. Moreover, hypermethylation is associated with developmental defects, such as gestational diabetes and Down’s syndrome [181, 182]. Kingsley et al. [183] also reported lower infant birth weight in association with maternal residence close to a major roadway was correlated with lower mean placental LINE-1 methylation. Moreover, seven CpG sites were significantly associated with residential proximity to major roadways. In a cohort study, exposure to PM2.5 during the first trimester was significantly associated with decreased (2.2%) global DNA methylation in placenta tissue [178] and was also significantly correlated with gene expression of S-adenosylmethionine (SAM), a key substrate involved in methyl group transfers [184]. Additionally, investigators reported significant placental DNA methylation in the promoter region of the leptin gene, an energy-regulating hormone involved in fetal growth and development, in association with maternal PM2.5 exposure [185]. Zhou et al. [186] linked air pollutant DNA methylation changes directly with oxidative stress, evidenced by significant associations with SOD2 promoter methylation levels in umbilical cord blood. In another cohort, Breton et al. [187] observed prenatal PM2.5 exposure was associated with altered DNA methylation in newborn blood in several gene promoters, some which were associated with cardio-respiratory health outcomes later in childhood. Additionally, maternal PM2.5 exposure has been correlated with overall placental DNA mutation rate, evidenced by an increased Alu rate (as an estimate of global methylation [188]) and altered DNA methylation in the promoter regions of the key DNA repair and tumor suppressor genes including APEX1, ERCC4, DAPK, and PARP1 [189, 190]. These findings suggest changes to fetal and neonatal DNA repair capacity may play a role in cancer risk later in life. Last, epigenetic modifications in placental mtDNA have been correlated with PM2.5 exposure. Epigenetic modifications in mtDNA have been associated with mitochondrial damage and dysfunction and are recognized as an etiological determinant in a variety of diseases, including diabetes, obesity, cardiovascular disease, and cancer [191]. Janssen et al. [192] reported in utero exposure to PM2.5 led to lower mtDNA content (as described in the section on oxidative stress), as well as modified the methylation level in the MT-RNR1 region.
Non-coding RNAs, which are not translated into protein, include microRNA (miRNAs), piwi-interacting RNA (piRNA), and long noncoding RNAs (lncRNAs), have been found to participate in various biological processes through interacting with transcriptional factors, repress complex and key regulatory proteins. MicroRNAs, which are endogenous single-stranded small non-coding RNAs of about 22 nucleotides, have been extensively investigated during the past decades and are found to play important regulatory roles in human diseases [193–195]. MiRNAs play a pivotal role in maintaining the healthy condition and regulating the redox state in lungs. Tsamou et al. [196] showed expression of miR-21, miR-146a, and miR-222 significantly decreased in the placental tissues following PM2.5 exposure during the second trimester of pregnancy, whereas expression of miR-20a and miR-21 increased in response to fine PM during the first trimester. Mir-21, which is an important regulator in vascular cell proliferation and apoptosis, was also significantly increased in response to diesel exhaust particle and metal-rich PM exposure in adults [197, 198]. Overall, additional research is needed to establish how these early miRNA and mRNA expression changes potentially influence health effects from both fine, as well as ultrafine, PM later in life.
Endocrine disruption
Evidence on the association between prenatal PM exposure and disorders of the endocrine system and hypothalamic-pituitary-adrenal (HPA) axis are only beginning to emerge. Janssen et al. reported maternal third-trimester exposure to PM2.5 was associated with differences in fetal thyroid hormone levels that may contribute to reduced birth weight and long-term health consequences for children [199]. Other mechanisms may involve PM effects on the HPA axis that alter the release of stress hormones like cortisol from the adrenal gland [200]. In a maternal cohort, Khamirchi et al. observed a significant positive association between gestational exposure to PM2.5 and cortisol levels in cord blood. The association for PM1 exposure was not statistically significant [201]. These results necessitate additional research to confirm findings and evaluate the potential mechanistic linkages between fine and ultrafine fine PM and endocrine-related effects. Moreover, refined measurement of potential endocrine disrupting compounds adsorbed to airborne particles [202] may shed additional light on heterogeneity between studies when only assessing particles based on size.
Preventive strategies
Green space to improve air quality
The impact of green space on air pollution exposure and mitigation of health effects in communities has been increasingly studied [203, 204]. A recent report that reviewed the literature on green space, heat, and air pollution generally found that urban green spaces, including trees, parks, green roofs, and large natural space, provide significant health benefits for residents and improvements in air quality [205]. Investigators have also shown children living in areas with more trees have a lower prevalence of early childhood asthma [206]. Specific to prenatal exposure, Dadvand et al. [207] demonstrated lower levels of personal exposure to PM2.5 and nitric oxides among pregnant women residing in greener areas in Barcelona, Spain. While few studies have interrogated the potential for green space to reduce maternal and child health effects from fine and ultrafine PM, expansion of green space and access for pregnant women may help combat the adverse health outcomes in children. This is particularly important when looking through the lens of racial inequity in the USA, as studies show historically redlined neighborhoods are still associated with reduced present-day green space [208]. Moreover, racial minority and low socioeconomic status groups experience a disproportionate exposure to air pollution. Across the USA, Blacks are estimated to have a 1.54 times higher burden from PM-emitting facilities than the overall population [209]. Remarkably, the burden for Black Americans was estimated to be higher than the all races living in poverty (1.35 higher burden), indicating disparities based on race may be more pronounced than disparities on the basis of poverty. Thus, combatting racist zoning at the neighborhood and regional levels can help reduce environmental disparities and protect children’s health.
Nutritional interventions
Nutrition is well recognized as an important modifier along the exposure to disease continuum. Indeed, there is a rich literature related to cancer prevention through the intake of whole foods or simple extracts. The term “green” chemoprevention has been applied to a food-centered approach that may be sustainable in underserved populations [210]. Dietary activation of antioxidant signaling pathways has been proposed as a potential strategy to mitigate pollutant-induced oxidative stress-mediated disease pathogenesis, for instance cancer and chronic lung disease [210, 211]. Clinical trials with broccoli sprouts, rich in the chemoprotective compound glucoraphanin and its biologically active metabolite sulforaphane, in populations highly exposed to air pollution demonstrate the ability of antioxidant pathway activation via Nrf2 to increase metabolism and excretion of airborne pollutants in healthy adults [212]. Since air pollution is an environmental factor that often cannot be avoided, the ability to enhance detoxication of air pollutants and attenuate their associated health risks is of high value, especially in susceptible subgroups.
Studies highlight protective effects from maternal fish consumption, a rich source of omega-3-polusaturated fatty acids (PUFAs). Jedrychowski et al. [213] demonstrated higher maternal exposure to PM2.5 was associated with significantly lower infant birth weight; investigators observed greater deficits in infant weight in mothers who reported low fish intake, as compared to medium or high fish consumption. Similarly, in the same cohort, high prenatal PM2.5 coupled with postnatal environmental tobacco smoke exposure led to a significantly increased risk for infant eczema, an inflammatory-driven dermatitis [214]. However, when maternal fish intake was high, the risk of infant eczema decreased by 43%, indicating possible benefits of PUFAs for reducing allergic disease. Calderón-Garcidueñas et al. [215] has also reviewed the potential benefit of cocoa flavonols to mitigate PM-associated cognitive and metabolic effects. Flavanols are part of a class of polyphenols known to exert protective effects through their function as antioxidants. In a short-term cocoa intervention, children in the Mexico City Metropolitan Area received 30 grams of dark cocoa with 680 mg of total flavonols; 83% (15/18) of children showed a marginally significant individual improvement in one or both of the applied simple short memory tasks [216]. Moreover, plasma endothelin-1, a potent vasoconstrictor, significantly decreased in response to cocoa intake. It has also been suggested that cocoa and cocoa flavonoids may positively affect endothelial dysfunction and insulin resistance, both known to be associated with PM2.5 exposure [217]. Since maternal fine and ultrafine PM exposure has been linked with offspring metabolic disorders via mitochondrial oxidative stress [75], targeting these pathways may also potentially prevent metabolic dysfunction. In an analogous mouse model of maternal tobacco smoke, prenatal exposure led to glucose intolerance, hepatic steatosis, and mitochondrial oxidative stress in adult offspring. Maternal administration of mitoquinone mesylate (MitoQ), a mitochondrial-targeted antioxidant, reduced hepatic mitochondrial oxidative stress and improved markers of mitophagy and mitochondrial biogenesis in offspring [218].
Findings from additional animal models also support the role of antioxidants to mitigate effects in offspring following prenatal PM2.5 exposure. Using elegant study design, Repreich et al. [103] demonstrated protection conferred from endotoxin (LPS) on offspring OVA-induced asthma was erased if dams were co-exposed to diesel exhaust PM during pregnancy. This effect was dependent on IFN-γ, and maternal treatment with the antioxidant N-acetyl cysteine (NAC) reversed the IFN-γ-dependent asthma risk in offspring, thereby re-conferring protection. Liu et al. [170] also demonstrated immune protective effects from quercetin, a plant-derived polyphenol, treatment during pregnancy. In this model, gestational PM2.5 exposure led to significantly altered T-lymphocyte subsets and increased markers of systemic inflammation and oxidative stress in peripheral blood of pregnant mice. In the quercetin intervention groups, higher doses of supplementation protected the dams against these adverse effects. In an ICR mouse model, gestational exposure to carbon black nanoparticles dose-dependently induced offspring astrogliosis, evidenced by increased reactive astrocytes with glial fibrillary acidic protein (GFAP) and aquaporin-4 overexpression [219]. Maternal antioxidant supplementation with the NAC suppressed GFAP overexpression in offspring, but did not suppress aquaporin-4 overexpression. Interestingly, maternal ascorbic acid (vitamin C) administration did not suppress, but rather slightly enhanced the expression of GFAP and aquaporin-4. These findings emphasize the importance of scrutinizing interventions in vivo, including combined effects, prior to clinical recommendations. This is particularly important in regards to safety of nutrition recommendation and use of dietary supplements during pregnancy. For instance, researchers have reported positive findings in in vitro models showing tea polyphenols ameliorate adverse effects of PM2.5 [220]. However, precaution has been advised for the consumption of tea polyphenols and others during late pregnancy based on possible adverse perinatal effects, particularly fetal ductus arteriosus [221].
Folic acid (a B vitamin) is routinely recommended based on evidence that supplementation can prevent neural tube defects [222]. A few studies have investigated the potential for folic acid to mitigate PM-induced health effects in offspring. In a mouse model, B-vitamin supplementation (folic acid, vitamin B6 and vitamin B12) significantly alleviated neurobehavioral impairment in offspring exposed to PM2.5 during gestation [223]. Moreover, supplementation increased neurogenesis and reduced synaptic loss and mitochondrial damage in offspring hippocampus. Pro-inflammatory cytokines and oxidative stress-related genes were also downregulated. Since folic acid is an important methyl donor, which is critical to numerous cellular functions, including DNA methylation, the role of folic acid supplementation on DNA methylation changes in the context of PM exposure have been recently interrogated. In a developmental zebrafish model, folic acid was shown to protect against PM2.5-induced cardiac toxicity in embryos [224]. Genomic-wide DNA methylation analysis demonstrated both hypo- and hyper-methylation changes in CCGG sites exposed to fine PM that were attenuated by folic acid supplementation [225]. These data suggest folic acid supplementation may protect against developmental cardiac toxicity by mitigating PM2.5-induced DNA methylation changes. Further research is required to confirm the protective capacity of folic acid supplementation against PM-induced health effects following developmental exposure.
Conclusions
In summary, fine and ultrafine PM are important components of ambient air pollution. Developmental exposure is associated with a wide-range of offspring health effects. A large body of observational studies in exposed human populations over decades highlight associations between prenatal fine PM exposure and adverse birth outcomes, namely, low birth weight, respiratory diseases, and adverse neurodevelopment. Recent studies report associations between prenatal fine PM exposure and immune system and metabolic alterations. The most recent State of the Global Air Report [1] for the first time included air pollution-related deaths mediated by low birth weight and preterm birth, which accounted for 20% of neonatal deaths worldwide (1/3 related to PM2.5 exposure). Imagine the effect on global morbidity and mortality if all adverse outcomes were to be included.
Additionally, a variety of experimental approaches demonstrate dose-response gradients and support causal associations between developmental fine and ultrafine PM exposure and several adverse outcomes in offspring. Detailed biological mechanisms of action established through molecular epidemiology studies and experimental models support the direct translocation of ultrafine particles across the placental and indirect mechanisms of action, including systemic oxidative stress, inflammation, and epigenetic changes. Additional mechanisms, like how early life air pollution exposure alters the infant microbiome and leads to adverse health outcomes are also beginning to emerge [226] and require increased understanding of how these changes may inform the development of clinical/nutritional strategies.
Overall, improved understanding of the plethora of health impacts stemming from developmental exposure to fine and ultrafine PM supports public health policies that reduce particulate air pollution, particularly for this sensitive population and disproportionally exposed groups. Continued research on health effects of ultrafine PM, source-specific effects, and the effectiveness of mitigation strategies in addition to reducing exposure when policy lags, will aid in reduced health effects in children and overall improved societal outcomes.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
Conceptualization – RZ, NMJ; Methodology and Data Curation – NMJ, NH; Writing Original Draft – NMJ, ARH, JCB, CL, DP, NH, RS, YL, JC, YT; Writing Review and Editing – NMJ, NH. The authors read and approved the final manuscript.
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. N. Johnson, A. Rodrigues Hoffmann, J. Behlen, C. Lau, D. Pendleton, N. Harvey, Y. Li, and R. Zhang were supported, in part, by the National Institute of Environmental Sciences (NIEHS) (ES028866). D. Pendleton and R. Shore were supported by T32 ES026568.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Natalie M. Johnson, Email: nmjohnson@tamu.edu
Aline Rodrigues Hoffmann, Email: arodrigues@cvm.tamu.edu.
Jonathan C. Behlen, Email: jbehlen@cvm.tamu.edu
Carmen Lau, Email: clau@cvm.tamu.edu.
Drew Pendleton, Email: drew967@tamu.edu.
Navada Harvey, Email: nharvey@tamu.edu.
Ross Shore, Email: rs4904@tamu.edu.
Yixin Li, Email: yxli11@tamu.edu.
Jingshu Chen, Email: jichen@cvm.tamu.edu.
Yanan Tian, Email: ytian@cvm.tamu.edu.
Renyi Zhang, Email: renyi-zhang@tamu.edu.
References
- 1.State of global air 2019. . In: Health Effects Institute Boston; 2019: No. 2578-6873.
- 2.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrante G, Antona R, Malizia V, Montalbano L, Corsello G, La Grutta S. Smoke exposure as a risk factor for asthma in childhood: a review of current evidence. Allergy Asthma Proc. 2014;35(6):454–461. doi: 10.2500/aap.2014.35.3789. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Tang Y, Song X, Lazar L, Li Z, Zhao J. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol Environ Saf. 2019;169:248–254. doi: 10.1016/j.ecoenv.2018.10.109. [DOI] [PubMed] [Google Scholar]
- 5.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: a systematic review. Environ Res. 2017;159:519–530. doi: 10.1016/j.envres.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Clifford A, Lang L, Chen R, Anstey KJ, Seaton A. Exposure to air pollution and cognitive functioning across the life course--a systematic literature review. Environ Res. 2016;147:383–398. doi: 10.1016/j.envres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Lim CC, Thurston GD. Air pollution, oxidative stress, and diabetes: a life course epidemiologic perspective. Curr Diab Rep. 2019;19(8):58. doi: 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne-Sturges DC, Marty MA, Perera F, Miller MD, Swanson M, Ellickson K, Cory-Slechta DA, Ritz B, Balmes J, Anderko L, et al. Healthy air, healthy brains: advancing air pollution policy to protect children’s health. Am J Public Health. 2019;109(4):550–554. doi: 10.2105/AJPH.2018.304902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Liu Y, Chen Y, Yao C, Che Z, Cao J. Maternal exposure to fine particulate matter (PM2.5) and pregnancy outcomes: a meta-analysis. Environ Sci Pollut Res Int. 2015;22(5):3383–3396. doi: 10.1007/s11356-014-3458-7. [DOI] [PubMed] [Google Scholar]
- 12.Lamichhane DK, Leem JH, Lee JY, Kim HC. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol. 2015;30:e2015011. doi: 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun X, Luo X, Zhao C, Zhang B, Tao J, Yang Z, Ma W, Liu T. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ Pollut. 2016;211:38–47. doi: 10.1016/j.envpol.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 14.DeFranco E, Moravec W, Xu F, Hall E, Hossain M, Haynes EN, Muglia L, Chen A. Exposure to airborne particulate matter during pregnancy is associated with preterm birth: a population-based cohort study. Environ Health. 2016;15:6. doi: 10.1186/s12940-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Percy Z, DeFranco E, Xu F, Hall ES, Haynes EN, Jones D, Muglia LJ, Chen A. Trimester specific PM2.5 exposure and fetal growth in Ohio, 2007-2010. Environ Res. 2019;171:111–118. doi: 10.1016/j.envres.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich DQ, Liu K, Zhang J, Thurston SW, Stevens TP, Pan Y, Kane C, Weinberger B, Ohman-Strickland P, Woodruff TJ, et al. Differences in birth weight associated with the 2008 Beijing Olympics air pollution reduction: results from a natural experiment. Environ Health Perspect. 2015;123(9):880–887. doi: 10.1289/ehp.1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddika N, Balogun HA, Amegah AK, Jaakkola JJ. Prenatal ambient air pollution exposure and the risk of stillbirth: systematic review and meta-analysis of the empirical evidence. Occup Environ Med. 2016;73(9):573–581. doi: 10.1136/oemed-2015-103086. [DOI] [PubMed] [Google Scholar]
- 18.DeFranco E, Hall E, Hossain M, Chen A, Haynes EN, Jones D, Ren S, Lu L, Muglia L. Air pollution and stillbirth risk: exposure to airborne particulate matter during pregnancy is associated with fetal death. Plos One. 2015;10(3):e0120594. doi: 10.1371/journal.pone.0120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EA MI, Gehring U, Molter A, Fuertes E, Klumper C, Kramer U, Quass U, Hoffmann B, Gascon M, Brunekreef B, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect. 2014;122(1):107–113. doi: 10.1289/ehp.1306755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Planell-Saguer M, Lovinsky-Desir S, Miller RL. Epigenetic regulation: the interface between prenatal and early-life exposure and asthma susceptibility. Environ Mol Mutagen. 2014;55(3):231–243. doi: 10.1002/em.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rychlik KA, FCM S. Environmental exposures during pregnancy: mechanistic effects on immunity. Birth Defects Res. 2019;111(4):178–196. doi: 10.1002/bdr2.1469. [DOI] [PubMed] [Google Scholar]
- 22.Baiz N, Slama R, Bene MC, Charles MA, Kolopp-Sarda MN, Magnan A, Thiebaugeorges O, Faure G, Annesi-Maesano I. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth. 2011;11:87. doi: 10.1186/1471-2393-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herr CE, Dostal M, Ghosh R, Ashwood P, Lipsett M, Pinkerton KE, Sram R, Hertz-Picciotto I. Air pollution exposure during critical time periods in gestation and alterations in cord blood lymphocyte distribution: a cohort of livebirths. Environ Health. 2010;9:46. doi: 10.1186/1476-069X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobak M, Leon DA. The effect of air pollution on infant mortality appears specific for respiratory causes in the postneonatal period. Epidemiology. 1999;10(6):666–670. doi: 10.1097/00001648-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Ha SU, Basnet R. A review of epidemiological research on adverse neurological effects of exposure to ambient air pollution. Front Public Health. 2016;4:157. doi: 10.3389/fpubh.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, Lawler C, Davidson L, Daniels N, Newschaffer C, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. Plos One. 2016;11(9):e0161851. doi: 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guxens M, Lubczynska MJ, Muetzel RL, Dalmau-Bueno A, VWV J, Hoek G, van der Lugt A, Verhulst FC, White T, Brunekreef B, et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry. 2018;84(4):295–303. doi: 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Mortamais M, Pujol J, Martinez-Vilavella G, Fenoll R, Reynes C, Sabatier R, Rivas I, Forns J, Vilor-Tejedor N, Alemany S, et al. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ Res. 2019;178:108734. doi: 10.1016/j.envres.2019.108734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas I, Basagana X, Cirach M, Lopez-Vicente M, Suades-Gonzalez E, Garcia-Esteban R, Alvarez-Pedrerol M, Dadvand P, Sunyer J. Association between early life exposure to air pollution and working memory and attention. Environ Health Perspect. 2019;127(5):57002. doi: 10.1289/EHP3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. 2017;(288):1–8. [PubMed]
- 31.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–591. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gingras V, Hivert MF, Oken E. Early-life exposures and risk of diabetes mellitus and obesity. Curr Diab Rep. 2018;18(10):89. doi: 10.1007/s11892-018-1050-0. [DOI] [PubMed] [Google Scholar]
- 34.Alderete TL, Song AY, Bastain T, Habre R, Toledo-Corral CM, Salam MT, Lurmann F, Gilliland FD, Breton CV. Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight. Pediatr Obes. 2018;13(6):348–356. doi: 10.1111/ijpo.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. Longitudinal associations between ambient air pollution with insulin sensitivity, beta-cell function, and adiposity in Los Angeles Latino children. Diabetes. 2017;66(7):1789–1796. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, Coull BA, Zanobetti A, Gillman MW, Gold DR, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26(1):43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, Koutrakis P, Schwartz JD, Zanobetti A, Mantzoros CS, et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes. 2017;12(1):48–57. doi: 10.1111/ijpo.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleisch AF, Aris IM, Rifas-Shiman SL, Coull BA, Luttmann-Gibson H, Koutrakis P, Schwartz JD, Kloog I, Gold DR, Oken E. Prenatal exposure to traffic pollution and childhood body mass index trajectory. Front Endocrinol (Lausanne) 2018;9:771. doi: 10.3389/fendo.2018.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, von Berg A, Koletzko S, Bauer CP, Heinrich J. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56(8):1696–1704. doi: 10.1007/s00125-013-2925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiering E, Markevych I, Bruske I, Fuertes E, Kratzsch J, Sugiri D, Hoffmann B, von Berg A, Bauer CP, Koletzko S, et al. Associations of residential long-term air pollution exposures and satellite-derived greenness with insulin resistance in German adolescents. Environ Health Perspect. 2016;124(8):1291–1298. doi: 10.1289/ehp.1509967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moody EC, Cantoral A, Tamayo-Ortiz M, Pizano-Zarate ML, Schnaas L, Kloog I, Oken E, Coull B, Baccarelli A, Tellez-Rojo MM, et al. Association of prenatal and perinatal exposures to particulate matter with changes in hemoglobin A1c levels in children aged 4 to 6 years. JAMA Netw Open. 2019;2(12):e1917643. doi: 10.1001/jamanetworkopen.2019.17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Mueller NT, Wang H, Hong X, Appel LJ, Wang X. Maternal exposure to ambient particulate matter </=2.5 microm during pregnancy and the risk for high blood pressure in childhood. Hypertension. 2018;72(1):194–201. doi: 10.1161/HYPERTENSIONAHA.117.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sears CG, Braun JM, Ryan PH, Xu Y, Werner EF, Lanphear BP, Wellenius GA. The association of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy in the HOME study cohort. Environ Int. 2018;121(Pt 1):574–581. doi: 10.1016/j.envint.2018.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira G, Haggar F, Shand AW, Bower C, Cook A, Nassar N. Association between pre-eclampsia and locally derived traffic-related air pollution: a retrospective cohort study. J Epidemiol Community Health. 2013;67(2):147–152. doi: 10.1136/jech-2011-200805. [DOI] [PubMed] [Google Scholar]
- 45.Wu M, Ries JJ, Proietti E, Vogt D, Hahn S, Hoesli I. Development of late-onset preeclampsia in association with road densities as a proxy for traffic-related air pollution. Fetal Diagn Ther. 2016;39(1):21–27. doi: 10.1159/000381802. [DOI] [PubMed] [Google Scholar]
- 46.Ohlwein S, Kappeler R, Kutlar Joss M, Kunzli N, Hoffmann B. Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Public Health. 2019;64(4):547–559. doi: 10.1007/s00038-019-01202-7. [DOI] [PubMed] [Google Scholar]
- 47.Wright RJ, Coull BA. Small but mighty: prenatal ultrafine particle exposure linked to childhood asthma incidence. Am J Respir Crit Care Med. 2019;199(12):1448–1450. doi: 10.1164/rccm.201903-0506ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavigne E, Donelle J, Hatzopoulou M, Van Ryswyk K, van Donkelaar A, Martin RV, Chen H, Stieb DM, Gasparrini A, Crighton E, et al. Spatiotemporal variations in ambient ultrafine particles and the incidence of childhood asthma. Am J Respir Crit Care Med. 2019;199(12):1487–1495. doi: 10.1164/rccm.201810-1976OC. [DOI] [PubMed] [Google Scholar]
- 49.Wright RJ, Hsu HL, Chiu YM, Coull BA, Simon MC, Hudda N, Schwartz J, Kloog I, Durant JL: Prenatal ambient ultrafine particle exposure and childhood asthma in the northeastern United States. Am J Respir Crit Care Med. 2021. 10.1164/rccm.202010-3743OC. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 50.Pan H, Deutsch GH, Wert SE, Ontology S, Consortium NMAoLDP Comprehensive anatomic ontologies for lung development: a comparison of alveolar formation and maturation within mouse and human lung. J Biomed Semantics. 2019;10(1):18. doi: 10.1186/s13326-019-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen LC, Lippmann M. Inhalation toxicology methods: the generation and characterization of exposure atmospheres and inhalational exposures. Curr Protoc Toxicol. 2015;63:24 24 21–24 24 23. doi: 10.1002/0471140856.tx2404s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauad T, Rivero DH, de Oliveira RC, Lichtenfels AJ, Guimaraes ET, de Andre PA, Kasahara DI, Bueno HM, Saldiva PH. Chronic exposure to ambient levels of urban particles affects mouse lung development. Am J Respir Crit Care Med. 2008;178(7):721–728. doi: 10.1164/rccm.200803-436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y, Zhang JJ, Li Z, Gow A, Chung KF, Hu M, Sun Z, Zeng L, Zhu T, Jia G, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J. 2016;30(6):2115–2122. doi: 10.1096/fj.201500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen JL, Conrad K, Oberdorster G, Johnston CJ, Sleezer B, Cory-Slechta DA. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ Health Perspect. 2013;121(1):32–38. doi: 10.1289/ehp.1205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen JL, Liu X, Weston D, Prince L, Oberdorster G, Finkelstein JN, Johnston CJ, Cory-Slechta DA. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140(1):160–178. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen JL, Liu X, Pelkowski S, Palmer B, Conrad K, Oberdorster G, Weston D, Mayer-Proschel M, Cory-Slechta DA. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ Health Perspect. 2014;122(9):939–945. doi: 10.1289/ehp.1307984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, Conrad K, Mayer-Proschel M, Cory-Slechta DA. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017;59:140–154. doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cory-Slechta DA, Allen JL, Conrad K, Marvin E, Sobolewski M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology. 2018;69:217–231. doi: 10.1016/j.neuro.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sobolewski M, Anderson T, Conrad K, Marvin E, Klocke C, Morris-Schaffer K, Allen JL, Cory-Slechta DA. Developmental exposures to ultrafine particle air pollution reduces early testosterone levels and adult male social novelty preference: risk for children’s sex-biased neurobehavioral disorders. Neurotoxicology. 2018;68:203–211. doi: 10.1016/j.neuro.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klocke C, Allen JL, Sobolewski M, Mayer-Proschel M, Blum JL, Lauterstein D, Zelikoff JT, Cory-Slechta DA. Neuropathological consequences of gestational exposure to concentrated ambient fine and ultrafine particles in the mouse. Toxicol Sci. 2017;156(2):492–508. doi: 10.1093/toxsci/kfx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klocke C, Sherina V, Graham UM, Gunderson J, Allen JL, Sobolewski M, Blum JL, Zelikoff JT, Cory-Slechta DA. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal Toxicol. 2018;30(9-10):381–396. doi: 10.1080/08958378.2018.1533053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Church JS, Tijerina PB, Emerson FJ, Coburn MA, Blum JL, Zelikoff JT, Schwartzer JJ. Perinatal exposure to concentrated ambient particulates results in autism-like behavioral deficits in adult mice. Neurotoxicology. 2018;65:231–240. doi: 10.1016/j.neuro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorr MW, Velten M, Nelin TD, Youtz DJ, Sun Q, Wold LE. Early life exposure to air pollution induces adult cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;307(9):H1353–H1360. doi: 10.1152/ajpheart.00526.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanwar V, Gorr MW, Velten M, Eichenseer CM, Long VP, 3rd, Bonilla IM, Shettigar V, Ziolo MT, Davis JP, Baine SH et al: In utero particulate matter exposure produces heart failure, electrical remodeling, and epigenetic changes at adulthood. J Am Heart Assoc. 2017;6(4):e005796. 10.1161/JAHA.117.005796. [DOI] [PMC free article] [PubMed]
- 66.Chen M, Wang X, Hu Z, Zhou H, Xu Y, Qiu L, Qin X, Zhang Y, Ying Z. Programming of mouse obesity by maternal exposure to concentrated ambient fine particles. Part Fibre Toxicol. 2017;14(1):20. doi: 10.1186/s12989-017-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang R, Wang G, Guo S, Zamora ML, Ying Q, Lin Y, Wang W, Hu M, Wang Y. Formation of urban fine particulate matter. Chem Rev. 2015;115(10):3803–3855. doi: 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- 68.Rychlik KA, Secrest JR, Lau C, Pulczinski J, Zamora ML, Leal J, Langley R, Myatt LG, Raju M, Chang RC, et al. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc Natl Acad Sci U S A. 2019;116(9):3443–3448. doi: 10.1073/pnas.1816103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu G, Brown J, Zamora ML, Miller A, Satterfield MC, Meininger CJ, Steinhauser CB, Johnson GA, Burghardt RC, Bazer FW, et al. Adverse organogenesis and predisposed long-term metabolic syndrome from prenatal exposure to fine particulate matter. Proc Natl Acad Sci U S A. 2019;116(24):11590–11595. doi: 10.1073/pnas.1902925116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo S, Hu M, Zamora ML, Peng J, Shang D, Zheng J, Du Z, Wu Z, Shao M, Zeng L, et al. Elucidating severe urban haze formation in China. Proc Natl Acad Sci U S A. 2014;111(49):17373–17378. doi: 10.1073/pnas.1419604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vejerano EP, Rao G, Khachatryan L, Cormier SA, Lomnicki S. Environmentally persistent free radicals: insights on a new class of pollutants. Environ Sci Technol. 2018;52(5):2468–2481. doi: 10.1021/acs.est.7b04439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thevenot PT, Saravia J, Jin N, Giaimo JD, Chustz RE, Mahne S, Kelley MA, Hebert VY, Dellinger B, Dugas TR, et al. Radical-containing ultrafine particulate matter initiates epithelial-to-mesenchymal transitions in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;48(2):188–197. doi: 10.1165/rcmb.2012-0052OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saravia J, You D, Thevenot P, Lee GI, Shrestha B, Lomnicki S, Cormier SA. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014;7(3):694–704. doi: 10.1038/mi.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee GI, Saravia J, You D, Shrestha B, Jaligama S, Hebert VY, Dugas TR, Cormier SA. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part Fibre Toxicol. 2014;11:57. doi: 10.1186/s12989-014-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stephenson EJ, Ragauskas A, Jaligama S, Redd JR, Parvathareddy J, Peloquin MJ, Saravia J, Han JC, Cormier SA, Bridges D. Exposure to environmentally persistent free radicals during gestation lowers energy expenditure and impairs skeletal muscle mitochondrial function in adult mice. Am J Physiol Endocrinol Metab. 2016;310(11):E1003–E1015. doi: 10.1152/ajpendo.00521.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jaligama S, Saravia J, You D, Yadav N, Lee GI, Shrestha B, Cormier SA. Regulatory T cells and IL10 suppress pulmonary host defense during early-life exposure to radical containing combustion derived ultrafine particulate matter. Respir Res. 2017;18(1):15. doi: 10.1186/s12931-016-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.dela Cruz AL, Gehling W, Lomnicki S, Cook R, Dellinger B. Detection of environmentally persistent free radicals at a superfund wood treating site. Environ Sci Technol. 2011;45(15):6356–6365. doi: 10.1021/es2012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamada K, Suzaki Y, Leme A, Ito T, Miyamoto K, Kobzik L, Kimura H. Exposure of pregnant mice to an air pollutant aerosol increases asthma susceptibility in offspring. J Toxicol Environ Health A. 2007;70(8):688–695. doi: 10.1080/15287390600974692. [DOI] [PubMed] [Google Scholar]
- 79.Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, Shih JC, Berhane K, McConnell R, Sioutas C, et al. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. Plos One. 2013;8(5):e64128. doi: 10.1371/journal.pone.0064128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woodward NC, Crow AL, Zhang Y, Epstein S, Hartiala J, Johnson R, Kocalis H, Saffari A, Sankaranarayanan I, Akbari O, et al. Exposure to nanoscale particulate matter from gestation to adulthood impairs metabolic homeostasis in mice. Sci Rep. 2019;9(1):1816. doi: 10.1038/s41598-018-37704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, Wallin H. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Part Fibre Toxicol. 2008;5:3. doi: 10.1186/1743-8977-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corson L, Zhu H, Quan C, Grunig G, Ballaney M, Jin X, Perera FP, Factor PH, Chen LC, Miller RL. Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice. Allergy Asthma Clin Immunol. 2010;6(1):7. doi: 10.1186/1710-1492-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008;38(1):57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2014;134(1):63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong X, Liu C, Chen X, Song Y, Wang Q, Wang P, Hu D. Maternal exposure to airborne particulate matter causes postnatal immunological dysfunction in mice offspring. Toxicology. 2013;306:59–67. doi: 10.1016/j.tox.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 86.Chen M, Liang S, Qin X, Zhang L, Qiu L, Chen S, Hu Z, Xu Y, Wang W, Zhang Y, et al. Prenatal exposure to diesel exhaust PM2.5 causes offspring beta cell dysfunction in adulthood. Am J Physiol Endocrinol Metab. 2018;315(1):E72–E80. doi: 10.1152/ajpendo.00336.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen M, Liang S, Zhou H, Xu Y, Qin X, Hu Z, Wang X, Qiu L, Wang W, Zhang Y, et al. Prenatal and postnatal mothering by diesel exhaust PM2.5-exposed dams differentially program mouse energy metabolism. Part Fibre Toxicol. 2017;14(1):3. doi: 10.1186/s12989-017-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa DL, Lehmann JR, Winsett D, Richards J, Ledbetter AD, Dreher KL. Comparative pulmonary toxicological assessment of oil combustion particles following inhalation or instillation exposure. Toxicol Sci. 2006;91(1):237–246. doi: 10.1093/toxsci/kfj123. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Wang L, Wang F, Li C. Effect of fine particulate matter (PM2.5) on rat placenta pathology and perinatal outcomes. Med Sci Monit. 2016;22:3274–3280. doi: 10.12659/MSM.897808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie P, Zhao C, Huang W, Yong T, ACK C, He K, Chen X, Cai Z. Prenatal exposure to ambient fine particulate matter induces dysregulations of lipid metabolism in adipose tissue in male offspring. Sci Total Environ. 2019;657:1389–1397. doi: 10.1016/j.scitotenv.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida S, Takano H, Nishikawa M, Miao H, Ichinose T. Effects of fetal exposure to urban particulate matter on the immune system of male mouse offspring. Biol Pharm Bull. 2012;35(8):1238–1243. doi: 10.1248/bpb.b110708. [DOI] [PubMed] [Google Scholar]
- 92.Morales-Rubio RA, Alvarado-Cruz I, Manzano-Leon N, Andrade-Oliva MD, Uribe-Ramirez M, Quintanilla-Vega B, Osornio-Vargas A, De Vizcaya-Ruiz A. In utero exposure to ultrafine particles promotes placental stress-induced programming of renin-angiotensin system-related elements in the offspring results in altered blood pressure in adult mice. Part Fibre Toxicol. 2019;16(1):7. doi: 10.1186/s12989-019-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miranda RA, da Silva Franco CC, Previate C, Alves VS, Francisco FA, Moreira VM, de Moraes AMP, Gomes RM, Picinato MC, Natali MRM et al: Particulate matter exposure during perinatal life results in impaired glucose metabolism in adult male rat offspring. Cell Physiol Biochem 2018, 49(1):395-405. [DOI] [PubMed]
- 94.Tang W, Huang S, Du L, Sun W, Yu Z, Zhou Y, Chen J, Li X, Li X, Yu B, et al. Expression of HMGB1 in maternal exposure to fine particulate air pollution induces lung injury in rat offspring assessed with micro-CT. Chem Biol Interact. 2018;280:64–69. doi: 10.1016/j.cbi.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 95.Shang Y, Sun Q. Particulate air pollution: major research methods and applications in animal models. Environ Dis. 2018;3(3):57–62. doi: 10.4103/ed.ed_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ema M, Naya M, Horimoto M, Kato H. Developmental toxicity of diesel exhaust: a review of studies in experimental animals. Reprod Toxicol. 2013;42:1–17. doi: 10.1016/j.reprotox.2013.06.074. [DOI] [PubMed] [Google Scholar]
- 97.Yokota S, Oshio S, Moriya N, Takeda K. Social isolation-induced territorial aggression in male offspring is enhanced by exposure to diesel exhaust during pregnancy. Plos One. 2016;11(2):e0149737. doi: 10.1371/journal.pone.0149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang YC, Cole TB, Costa LG. Prenatal and early-life diesel exhaust exposure causes autism-like behavioral changes in mice. Part Fibre Toxicol. 2018;15(1):18. doi: 10.1186/s12989-018-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weldy CS, Liu Y, Chang YC, Medvedev IO, Fox JR, Larson TV, Chien WM, Chin MT. In utero and early life exposure to diesel exhaust air pollution increases adult susceptibility to heart failure in mice. Part Fibre Toxicol. 2013;10(1):59. doi: 10.1186/1743-8977-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harrigan J, Ravi D, Ricks J, Rosenfeld ME. In utero exposure of hyperlipidemic mice to diesel exhaust: lack of effects on atherosclerosis in adult offspring fed a regular chow diet. Cardiovasc Toxicol. 2017;17(4):417–425. doi: 10.1007/s12012-017-9399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsukue N, Tsubone H, Suzuki AK. Diesel exhaust affects the abnormal delivery in pregnant mice and the growth of their young. Inhal Toxicol. 2002;14(6):635–651. doi: 10.1080/08958370290084548. [DOI] [PubMed] [Google Scholar]
- 102.Sharkhuu T, Doerfler DL, Krantz QT, Luebke RW, Linak WP, Gilmour MI. Effects of prenatal diesel exhaust inhalation on pulmonary inflammation and development of specific immune responses. Toxicol Lett. 2010;196(1):12–20. doi: 10.1016/j.toxlet.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 103.Reiprich M, Rudzok S, Schutze N, Simon JC, Lehmann I, Trump S, Polte T. Inhibition of endotoxin-induced perinatal asthma protection by pollutants in an experimental mouse model. Allergy. 2013;68(4):481–489. doi: 10.1111/all.12121. [DOI] [PubMed] [Google Scholar]
- 104.Wang P, You D, Saravia J, Shen H, Cormier SA. Maternal exposure to combustion generated PM inhibits pulmonary Th1 maturation and concomitantly enhances postnatal asthma development in offspring. Part Fibre Toxicol. 2013;10:29. doi: 10.1186/1743-8977-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El-Sayed YS, Shimizu R, Onoda A, Takeda K, Umezawa M. Carbon black nanoparticle exposure during middle and late fetal development induces immune activation in male offspring mice. Toxicology. 2015;327:53–61. doi: 10.1016/j.tox.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Paul E, Franco-Montoya ML, Paineau E, Angeletti B, Vibhushan S, Ridoux A, Tiendrebeogo A, Salome M, Hesse B, Vantelon D, et al. Pulmonary exposure to metallic nanomaterials during pregnancy irreversibly impairs lung development of the offspring. Nanotoxicology. 2017;11(4):484–495. doi: 10.1080/17435390.2017.1311381. [DOI] [PubMed] [Google Scholar]
- 107.de Barros Mendes Lopes T, Groth EE, Veras M, Furuya TK, de Souza Xavier Costa N, Ribeiro Junior G, Lopes FD, de Almeida FM, Cardoso WV, PHN S, et al. Pre- and postnatal exposure of mice to concentrated urban PM2.5 decreases the number of alveoli and leads to altered lung function at an early stage of life. Environ Pollut. 2018;241:511–520. doi: 10.1016/j.envpol.2018.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meyer-Martin H, Reuter S, Taube C. Mouse models of allergic airway disease. Methods Mol Biol. 2014;1193:127–141. doi: 10.1007/978-1-4939-1212-4_13. [DOI] [PubMed] [Google Scholar]
- 109.Gutierrez-Vazquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48(1):19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, Schram-Bijkerk D, Brunekreef B, van Hage M, Scheynius A, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117(4):817–823. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 111.Dysart MM, Galvis BR, Russell AG, Barker TH. Environmental particulate (PM2.5) augments stiffness-induced alveolar epithelial cell mechanoactivation of transforming growth factor beta. Plos One. 2014;9(9):e106821. doi: 10.1371/journal.pone.0106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, Takeda K. utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol. 2010;7:7. doi: 10.1186/1743-8977-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Onoda A, Takeda K, Umezawa M. Dose-dependent induction of astrocyte activation and reactive astrogliosis in mouse brain following maternal exposure to carbon black nanoparticle. Part Fibre Toxicol. 2017;14(1):4. doi: 10.1186/s12989-017-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kulas JA, Hettwer JV, Sohrabi M, Melvin JE, Manocha GD, Puig KL, Gorr MW, Tanwar V, MP MD, Wold LE, et al. In utero exposure to fine particulate matter results in an altered neuroimmune phenotype in adult mice. Environ Pollut. 2018;241:279–288. doi: 10.1016/j.envpol.2018.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klocke C, Allen JL, Sobolewski M, Blum JL, Zelikoff JT, Cory-Slechta DA. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology. 2018;65:196–206. doi: 10.1016/j.neuro.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woodward NC, Haghani A, Johnson RG, Hsu TM, Saffari A, Sioutas C, Kanoski SE, Finch CE, Morgan TE. Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Transl Psychiatry. 2018;8(1):261. doi: 10.1038/s41398-018-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cui J, Fu Y, Lu R, Bi Y, Zhang L, Zhang C, Aschner M, Li X, Chen R. Metabolomics analysis explores the rescue to neurobehavioral disorder induced by maternal PM2.5 exposure in mice. Ecotoxicol Environ Saf. 2019;169:687–695. doi: 10.1016/j.ecoenv.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 118.Morris-Schaffer K, Merrill A, Jew K, Wong C, Conrad K, Harvey K, Marvin E, Sobolewski M, Oberdorster G, Elder A, et al. Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Part Fibre Toxicol. 2019;16(1):10. doi: 10.1186/s12989-019-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loomes R, Hull L, WPL M. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56(6):466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 120.DJ DS, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bresgen N, Eckl PM. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules. 2015;5(2):808–847. doi: 10.3390/biom5020808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Z, Chadalapaka G, Ramesh A, Khoshbouei H, Maguire M, Safe S, Rhoades RE, Clark R, Jules G, McCallister M, et al. PAH particles perturb prenatal processes and phenotypes: protection from deficits in object discrimination afforded by dampening of brain oxidoreductase following in utero exposure to inhaled benzo(a)pyrene. Toxicol Sci. 2012;125(1):233–247. doi: 10.1093/toxsci/kfr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamanaka RB, Mutlu GM. Particulate matter air pollution: effects on the cardiovascular system. Front Endocrinol (Lausanne) 2018;9:680. doi: 10.3389/fendo.2018.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goodson JM, Weldy CS, JW MD, Liu Y, Bammler TK, Chien WM, Chin MT. In utero exposure to diesel exhaust particulates is associated with an altered cardiac transcriptional response to transverse aortic constriction and altered DNA methylation. FASEB J. 2017;31(11):4935–4945. doi: 10.1096/fj.201700032R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye Z, Lu X, Deng Y, Wang X, Zheng S, Ren H, Zhang M, Chen T, Jose PA, Yang J, et al. In utero exposure to fine particulate matter causes hypertension due to impaired renal dopamine D1 receptor in offspring. Cell Physiol Biochem. 2018;46(1):148–159. doi: 10.1159/000488418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53(4 Pt 1):503–514. [PubMed] [Google Scholar]
- 128.Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674(1-2):45–54. doi: 10.1016/j.mrgentox.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 129.Lo Sasso G, Schlage WK, Boue S, Veljkovic E, Peitsch MC, Hoeng J. The Apoe(-/-) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J Transl Med. 2016;14(1):146. doi: 10.1186/s12967-016-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 131.Saenen ND, Martens DS, Neven KY, Alfano R, Bove H, Janssen BG, Roels HA, Plusquin M, Vrijens K, Nawrot TS. Air pollution-induced placental alterations: an interplay of oxidative stress, epigenetics, and the aging phenotype? Clin Epigenetics. 2019;11(1):124. doi: 10.1186/s13148-019-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan Q, Liew Z, Uppal K, Cui X, Ling C, Heck JE, von Ehrenstein OS, Wu J, Walker DI, Jones DP, et al. Maternal serum metabolome and traffic-related air pollution exposure in pregnancy. Environ Int. 2019;130:104872. doi: 10.1016/j.envint.2019.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muoth C, Aengenheister L, Kucki M, Wick P, Buerki-Thurnherr T. Nanoparticle transport across the placental barrier: pushing the field forward! Nanomedicine (Lond) 2016;11(8):941–957. doi: 10.2217/nnm-2015-0012. [DOI] [PubMed] [Google Scholar]
- 134.Bove H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, Van Eyken P, Plusquin M, MBJ R, Ameloot M, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10(1):3866. doi: 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valentino SA, Tarrade A, Aioun J, Mourier E, Richard C, Dahirel M, Rousseau-Ralliard D, Fournier N, Aubriere MC, Lallemand MS, et al. Maternal exposure to diluted diesel engine exhaust alters placental function and induces intergenerational effects in rabbits. Part Fibre Toxicol. 2016;13(1):39. doi: 10.1186/s12989-016-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Veras MM, Damaceno-Rodrigues NR, Caldini EG, Maciel Ribeiro AA, Mayhew TM, Saldiva PH, Dolhnikoff M. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol Reprod. 2008;79(3):578–584. doi: 10.1095/biolreprod.108.069591. [DOI] [PubMed] [Google Scholar]
- 137.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9-10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 138.Erickson AC, Arbour L. The shared pathoetiological effects of particulate air pollution and the social environment on fetal-placental development. J Environ Public Health. 2014;2014:901017. doi: 10.1155/2014/901017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114(11):1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pardo M, Xu F, Shemesh M, Qiu X, Barak Y, Zhu T, Rudich Y. Nrf2 protects against diverse PM2.5 components-induced mitochondrial oxidative damage in lung cells. Sci Total Environ. 2019;669:303–313. doi: 10.1016/j.scitotenv.2019.01.436. [DOI] [PubMed] [Google Scholar]
- 143.Stejskalova L, Pavek P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr Pharm Biotechnol. 2011;12(5):715–730. doi: 10.2174/138920111795470994. [DOI] [PubMed] [Google Scholar]
- 144.Kensler TW, Wakabayashi N, Biswal S, et al. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 145.Yang SI, Kim BJ, Lee SY, Kim HB, Lee CM, Yu J, Kang MJ, Yu HS, Lee E, Jung YH, et al. Prenatal particulate matter/tobacco smoke increases infants’ respiratory infections: COCOA study. Allergy Asthma Immunol Res. 2015;7(6):573–582. doi: 10.4168/aair.2015.7.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol. 2008;128(3):366–373. doi: 10.1016/j.clim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 147.Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, Horwitz LD, Brechun N, Diaz-Sanchez D, Nel AE. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168(5):2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 148.Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27(20):7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8(1-2):88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 150.Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114(4):627–633. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7(3-4):395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 152.Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem Soc Trans. 2015;43(4):621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010;13(11):1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagiah S, Phulukdaree A, Naidoo D, Ramcharan K, Naidoo RN, Moodley D, Chuturgoon A. Oxidative stress and air pollution exposure during pregnancy: a molecular assessment. Hum Exp Toxicol. 2015;34(8):838–847. doi: 10.1177/0960327114559992. [DOI] [PubMed] [Google Scholar]
- 155.Ambroz A, Vlkova V, Rossner P, Jr, Rossnerova A, Svecova V, Milcova A, Pulkrabova J, Hajslova J, Veleminsky M, Jr, Solansky I, et al. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int J Hyg Environ Health. 2016;219(6):545–556. doi: 10.1016/j.ijheh.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 156.Ren C, Baccarelli A, Wilker E, Suh H, Sparrow D, Vokonas P, Wright R, Schwartz J. Lipid and endothelium-related genes, ambient particulate matter, and heart rate variability--the VA Normative Aging Study. J Epidemiol Community Health. 2010;64(1):49–56. doi: 10.1136/jech.2008.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.JGF H, Madhloum N, Saenen ND, Janssen BG, Penders J, Vanpoucke C, De Vivo I, Vrijens K, Nawrot TS. Prenatal particulate air pollution exposure and cord blood homocysteine in newborns: results from the ENVIRONAGE birth cohort. Environ Res. 2019;168:507–513. doi: 10.1016/j.envres.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 158.Saenen ND, Vrijens K, Janssen BG, Madhloum N, Peusens M, Gyselaers W, Vanpoucke C, Lefebvre W, Roels HA, Nawrot TS. Placental nitrosative stress and exposure to ambient air pollution during gestation: a population study. Am J Epidemiol. 2016;184(6):442–449. doi: 10.1093/aje/kww007. [DOI] [PubMed] [Google Scholar]
- 159.Rosa MJ, Just AC, Guerra MS, Kloog I, Hsu HL, Brennan KJ, Garcia AM, Coull B, Wright RJ, Tellez Rojo MM, et al. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ Int. 2017;98:198–203. doi: 10.1016/j.envint.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brunst KJ, Sanchez-Guerra M, Chiu YM, Wilson A, Coull BA, Kloog I, Schwartz J, Brennan KJ, Bosquet Enlow M, Wright RO, et al. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: effect modification by maternal lifetime trauma and child sex. Environ Int. 2018;112:49–58. doi: 10.1016/j.envint.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grevendonk L, Janssen BG, Vanpoucke C, Lefebvre W, Hoxha M, Bollati V, Nawrot TS. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ Health. 2016;15:10. doi: 10.1186/s12940-016-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martens DS, Cox B, Janssen BG, DBP C, Gasparrini A, Vanpoucke C, Lefebvre W, Roels HA, Plusquin M, Nawrot TS. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr. 2017;171(12):1160–1167. doi: 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosa MJ, Hsu HL, Just AC, Brennan KJ, Bloomquist T, Kloog I, Pantic I, Mercado Garcia A, Wilson A, Coull BA, et al. Association between prenatal particulate air pollution exposure and telomere length in cord blood: effect modification by fetal sex. Environ Res. 2019;172:495–501. doi: 10.1016/j.envres.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Viveros-Alcaraz M, Castillo-Castrejon M, Beltran-Montoya J, Brown DG, O'Neill MS. Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses. 2014;82(2):219–224. doi: 10.1016/j.mehy.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee PC, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011;22(4):524–531. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mihu D, Costin N, Mihu CM, Blaga LD, Pop RB. C-reactive protein, marker for evaluation of systemic inflammatory response in preeclampsia. Rev Med Chir Soc Med Nat Iasi. 2008;112(4):1019–1025. [PubMed] [Google Scholar]
- 167.Nachman RM, Mao G, Zhang X, Hong X, Chen Z, Soria CS, He H, Wang G, Caruso D, Pearson C, et al. Intrauterine inflammation and maternal exposure to ambient PM2.5 during preconception and specific periods of pregnancy: the Boston birth cohort. Environ Health Perspect. 2016;124(10):1608–1615. doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujimoto A, Tsukue N, Watanabe M, Sugawara I, Yanagisawa R, Takano H, Yoshida S, Takeda K. Diesel exhaust affects immunological action in the placentas of mice. Environ Toxicol. 2005;20(4):431–440. doi: 10.1002/tox.20129. [DOI] [PubMed] [Google Scholar]
- 169.de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, Veras MM, Furukawa LN, de Castro I, Saldiva PH, Heimann JC. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett. 2015;232(2):475–480. doi: 10.1016/j.toxlet.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 170.Liu W, Zhang M, Feng J, Fan A, Zhou Y, Xu Y: The influence of quercetin on maternal immunity, oxidative stress, and inflammation in mice with exposure of fine particulate matter during gestation. Int J Environ Res Public Health. 2017;14(6):592. 10.3390/ijerph14060592. [DOI] [PMC free article] [PubMed]
- 171.Veras MM, Guimaraes-Silva RM, Caldini EG, Saldiva PH, Dolhnikoff M, Mayhew TM. The effects of particulate ambient air pollution on the murine umbilical cord and its vessels: a quantitative morphological and immunohistochemical study. Reprod Toxicol. 2012;34(4):598–606. doi: 10.1016/j.reprotox.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 172.Song P, Li Z, Li X, Yang L, Zhang L, Li N, Guo C, Lu S, Wei Y: Transcriptome profiling of the lungs reveals molecular clock genes expression changes after chronic exposure to ambient air particles. Int J Environ Res Public Health. 2017;14(1):90. Published online 2017 Jan 18. 10.3390/ijerph14010090. [DOI] [PMC free article] [PubMed]
- 173.Ferrari L, Carugno M, Bollati V. Particulate matter exposure shapes DNA methylation through the lifespan. Clin Epigenet. 2019;11(1):129. doi: 10.1186/s13148-019-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Na Rev Genet. 2007;8(4):253. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hamm CA, Costa FF. Epigenomes as therapeutic targets. Pharmaco Ther. 2015;151:72–86. doi: 10.1016/j.pharmthera.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 176.Waddington CH: Organisers and genes. Organisers and genes 1940. https://www.amazon.com/Organisers-Genes-C-H-Waddington/dp/B000GU4IUM.
- 177.Vaiserman A. Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: a potential link to disease susceptibility? Clin Epigenet. 2015;7(1):96. doi: 10.1186/s13148-015-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, Fierens F, Penders J, Plusquin M, Gyselaers W. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10(1):22. doi: 10.1186/1743-8977-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23(8):413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 180.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta (BBA)-Rev Cancer. 2007;1775(1):138–162. doi: 10.1016/j.bbcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 181.Reichetzeder C, Putra SD, Pfab T, Slowinski T, Neuber C, Kleuser B, Hocher B. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenet. 2016;8(1):82. doi: 10.1186/s13148-016-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jin S, Lee YK, Lim YC, Zheng Z, Lin XM, Ng DP, Holbrook JD, Law HY, Kwek KY, Yeo GS. Global DNA hypermethylation in down syndrome placenta. Plos Genet. 2013;9(6):e1003515. doi: 10.1371/journal.pgen.1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kingsley SL, Eliot MN, Whitsel EA, Huang YT, Kelsey KT, Marsit CJ, Wellenius GA. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int. 2016;92-93:43–49. doi: 10.1016/j.envint.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maghbooli Z, Hossein-nezhad A, Adabi E, Asadollah-pour E, Sadeghi M, Mohammad-nabi S, Rad LZ, Hosseini A-aM, Radmehr M, Faghihi F: Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. Plos One 2018, 13(7):e0199772. [DOI] [PMC free article] [PubMed]
- 185.Saenen ND, Vrijens K, Janssen BG, Roels HA, Neven KY, Vanden Berghe W, Gyselaers W, Vanpoucke C, Lefebvre W, De Boever P. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIR ON AGE cohort. Environ Health Perspect. 2016;125(2):262–268. doi: 10.1289/EHP38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhou G, He T, Huang H, Feng F, Liu X, Li Z, Zhang Y, Ba Y. Prenatal ambient air pollution exposure and SOD2 promoter methylation in maternal and cord blood. Ecotoxicol Environ Saf. 2019;181:428–434. doi: 10.1016/j.ecoenv.2019.06.039. [DOI] [PubMed] [Google Scholar]
- 187.Breton CV, Gao L, Yao J, Siegmund KD, Lurmann F, Gilliland F. Particulate matter, the newborn methylome, and cardio-respiratory health outcomes in childhood. Environ Epigenet. 2016;2(2):dvw005. doi: 10.1093/eep/dvw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang AS, Estécio MR, Doshi K, Kondo Y, Tajara EH, JPJ I. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Neven KY, Saenen ND, Tarantini L, Janssen BG, Lefebvre W, Vanpoucke C, Bollati V, Nawrot TS. Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: an ENVIRONAGE cohort study. Lancet Planet Health. 2018;2(4):e174–e183. doi: 10.1016/S2542-5196(18)30049-4. [DOI] [PubMed] [Google Scholar]
- 190.Alvarado-Cruz I, Sánchez-Guerra M, Hernández-Cadena L, De Vizcaya-Ruiz A, Mugica V, Pelallo-Martínez NA, de Jesús Solís-Heredia M, Byun H-M, Baccarelli A, Quintanilla-Vega B. Increased methylation of repetitive elements and DNA repair genes is associated with higher DNA oxidation in children in an urbanized, industrial environment. Mut Res. 2017;813:27–36. doi: 10.1016/j.mrgentox.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 191.Wallace DC. Mitochondrial DNA mutations in disease and aging. Environ Mol Mutagenesis. 2010;51(5):440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- 192.Janssen BG, Byun H-M, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: an ENVIR ON AGE birth cohort study. Epigenetics. 2015;10(6):536–544. doi: 10.1080/15592294.2015.1048412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nana-Sinkam SP, Hunter MG, Nuovo GJ, Schmittgen TD, Gelinas R, Galas D, Marsh CB. Integrating the MicroRNome into the study of lung disease. Am J Respir Crit Care Med. 2009;179(1):4–10. doi: 10.1164/rccm.200807-1042PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Erson A, Petty E. MicroRNAs in development and disease. Clin Genet. 2008;74(4):296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 195.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 196.Tsamou M, Vrijens K, Madhloum N, Lefebvre W, Vanpoucke C, Nawrot TS. Air pollution-induced placental epigenetic alterations in early life: a candidate miRNA approach. Epigenetics. 2018;13(2):135–146. doi: 10.1080/15592294.2016.1155012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M, Pegoraro V, Motta V, Tarantini L, Cantone L. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect. 2010;118(6):763–768. doi: 10.1289/ehp.0901300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bourdon JA, Saber AT, Halappanavar S, Jackson PA, Wu D, Hougaard KS, Jacobsen NR, Williams A, Vogel U, Wallin H. Carbon black nanoparticle intratracheal installation results in large and sustained changes in the expression of miR-135b in mouse lung. Environ Mol Mutagenesis. 2012;53(6):462–468. doi: 10.1002/em.21706. [DOI] [PubMed] [Google Scholar]
- 199.Janssen BG, Saenen ND, Roels HA, Madhloum N, Gyselaers W, Lefebvre W, Penders J, Vanpoucke C, Vrijens K, Nawrot TS. Fetal thyroid function, birth weight, and in utero exposure to fine particle air pollution: a birth cohort study. Environ Health Perspect. 2017;125(4):699–705. doi: 10.1289/EHP508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Niu Y, Chen R, Xia Y, Cai J, Ying Z, Lin Z, Liu C, Chen C, Peng L, Zhao Z, et al. Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis. Environ Int. 2018;119:186–192. doi: 10.1016/j.envint.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 201.Khamirchi R, Moslem A, Agah J, Pozo OJ, Miri M, Dadvand P. Maternal exposure to air pollution during pregnancy and cortisol level in cord blood. Sci Total Environ. 2020;713:136622. doi: 10.1016/j.scitotenv.2020.136622. [DOI] [PubMed] [Google Scholar]
- 202.Quintana-Belmares RO, Krais AM, Esfahani BK, Rosas-Perez I, Mucs D, Lopez-Marure R, Bergman A, Alfaro-Moreno E. Phthalate esters on urban airborne particles: levels in PM10 and PM2.5 from Mexico City and theoretical assessment of lung exposure. Environ Res. 2018;161:439–445. doi: 10.1016/j.envres.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 203.Nieuwenhuijsen MJ, Gascon M, Martinez D, Ponjoan A, Blanch J, Garcia-Gil MDM, Ramos R, Foraster M, Mueller N, Espinosa A et al: Air pollution, noise, blue space, and green space and premature mortality in Barcelona: a mega cohort. Int J Environ Res Public Health. 2018;15(11):2405. 10.3390/ijerph15112405. [DOI] [PMC free article] [PubMed]
- 204.Bloemsma LD, Gehring U, Klompmaker JO, Hoek G, NAH J, Lebret E, Brunekreef B, Wijga AH. Green space, air pollution, traffic noise and cardiometabolic health in adolescents: the PIAMA birth cohort. Environ Int. 2019;131:104991. doi: 10.1016/j.envint.2019.104991. [DOI] [PubMed] [Google Scholar]
- 205.The impact of green space on heat and air pollution in urban communities: a meta-narrative systematic review. https://davidsuzuki.org/wp-content/uploads/2017/09/impact-green-space-heat-air-pollution-urban-communities.pdf. Accessed 10 Feb 2020.
- 206.Lovasi GS, Quinn JW, Neckerman KM, Perzanowski MS, Rundle A. Children living in areas with more street trees have lower prevalence of asthma. J Epidemiol Community Health. 2008;62(7):647–649. doi: 10.1136/jech.2007.071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dadvand P, de Nazelle A, Triguero-Mas M, Schembari A, Cirach M, Amoly E, Figueras F, Basagana X, Ostro B, Nieuwenhuijsen M. Surrounding greenness and exposure to air pollution during pregnancy: an analysis of personal monitoring data. Environ Health Perspect. 2012;120(9):1286–1290. doi: 10.1289/ehp.1104609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nardone A, Rudolph KE, Morello-Frosch R, Casey JA. Redlines and greenspace: the relationship between historical redlining and 2010 greenspace across the United States. Environ Health Perspect. 2021;129(1):17006. doi: 10.1289/EHP7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mikati I, Benson AF, Luben TJ, Sacks JD, Richmond-Bryant J. Disparities in distribution of particulate matter emission sources by race and poverty status. Am J Public Health. 2018;108(4):480–485. doi: 10.2105/AJPH.2017.304297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fahey JW, Talalay P, Kensler TW. Notes from the field: “green” chemoprevention as frugal medicine. Cancer Prev Res (Phila) 2012;5(2):179–188. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim Biophys Acta. 2012;1822(5):714–728. doi: 10.1016/j.bbadis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kensler TW, Ng D, Carmella SG, Chen M, Jacobson LP, Munoz A, Egner PA, Chen JG, Qian GS, Chen TY, et al. Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China. Carcinogenesis. 2012;33(1):101–107. doi: 10.1093/carcin/bgr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jedrychowski W, Perera F, Mrozek-Budzyn D, Flak E, Mroz E, Sochacka-Tatara E, Jacek R, Kaim I, Skolicki Z, Spengler JD. Higher fish consumption in pregnancy may confer protection against the harmful effect of prenatal exposure to fine particulate matter. Ann Nutr Metab. 2010;56(2):119–126. doi: 10.1159/000275918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jedrychowski W, Perera F, Maugeri U, Mrozek-Budzyn D, Miller RL, Flak E, Mroz E, Jacek R, Spengler JD. Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol. 2011;155(3):275–281. doi: 10.1159/000320376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Calderon-Garciduenas L, San Juan Chavez V, Vacaseydel-Aceves NB, Calderon-Sanchez R, Macias-Escobedo E, Frias C, Giacometto M, Velasquez L, Felix-Villarreal R, Martin JD, et al. Chocolate, air pollution and children’s neuroprotection: what cognition tools should be at hand to evaluate interventions? Front Pharmacol. 2016;7:232. doi: 10.3389/fphar.2016.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Calderon-Garciduenas L, Mora-Tiscareno A, Franco-Lira M, Cross JV, Engle R, Aragon-Flores M, Gomez-Garza G, Jewells V, Medina-Cortina H, Solorio E, et al. Flavonol-rich dark cocoa significantly decreases plasma endothelin-1 and improves cognition in urban children. Front Pharmacol. 2013;4:104. doi: 10.3389/fphar.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grassi D, Desideri G, Mai F, Martella L, De Feo M, Soddu D, Fellini E, Veneri M, Stamerra CA, Ferri C. Cocoa, glucose tolerance, and insulin signaling: cardiometabolic protection. J Agric Food Chem. 2015;63(45):9919–9926. doi: 10.1021/acs.jafc.5b00913. [DOI] [PubMed] [Google Scholar]
- 218.Li G, Chan YL, Sukjamnong S, Anwer AG, Vindin H, Padula M, Zakarya R, George J, Oliver BG, Saad S et al: A mitochondrial specific antioxidant reverses metabolic dysfunction and fatty liver induced by maternal cigarette smoke in mice. Nutrients. 2019;11(7):1669. Published online 2019 July 21. 10.3390/nu11071669. [DOI] [PMC free article] [PubMed]
- 219.Onoda A, Takeda K, Umezawa M. Pretreatment with N-acetyl cysteine suppresses chronic reactive astrogliosis following maternal nanoparticle exposure during gestational period. Nanotoxicology. 2017;11(8):1012–1025. doi: 10.1080/17435390.2017.1388864. [DOI] [PubMed] [Google Scholar]
- 220.Zhang Y, Darland D, He Y, Yang L, Dong X, Chang Y: Reduction of Pm2.5 toxicity on human alveolar epithelial cells A549 by tea polyphenols. J Food Biochem. 2018;42(3):e12496. 10.1111/jfbc.12496. Epub 2018 Jan 18. [DOI] [PMC free article] [PubMed]
- 221.Zielinsky P, Busato S. Prenatal effects of maternal consumption of polyphenol-rich foods in late pregnancy upon fetal ductus arteriosus. Birth Defects Res C Embryo Today. 2013;99(4):256–274. doi: 10.1002/bdrc.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Folic acid for the prevention of neural tube defects. American Academy of Pediatrics. Committee on Genetics. Pediatrics 1999, 104(2 Pt 1):325-327. [DOI] [PubMed]
- 223.Wang T, Zhang T, Sun L, Li W, Zhang C, Yu L, Guan Y, et al. Ecotoxicol Environ Saf. 2019;185:109686. doi: 10.1016/j.ecoenv.2019.109686. [DOI] [PubMed] [Google Scholar]
- 224.Yue C, Ji C, Zhang H, Zhang LW, Tong J, Jiang Y, Chen T. Protective effects of folic acid on PM2.5-induced cardiac developmental toxicity in zebrafish embryos by targeting AhR and Wnt/beta-catenin signal pathways. Environ Toxicol. 2017;32(10):2316–2322. doi: 10.1002/tox.22448. [DOI] [PubMed] [Google Scholar]
- 225.Jiang Y, Li J, Ren F, Ji C, Aniagu S, Chen T. PM2.5-induced extensive DNA methylation changes in the heart of zebrafish embryos and the protective effect of folic acid. Environ Pollut. 2019;255(Pt 3):113331. doi: 10.1016/j.envpol.2019.113331. [DOI] [PubMed] [Google Scholar]
- 226.Valles Y, Francino MP. Air pollution, early life microbiome, and development. Curr Environ Health Rep. 2018;5(4):512–521. doi: 10.1007/s40572-018-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.