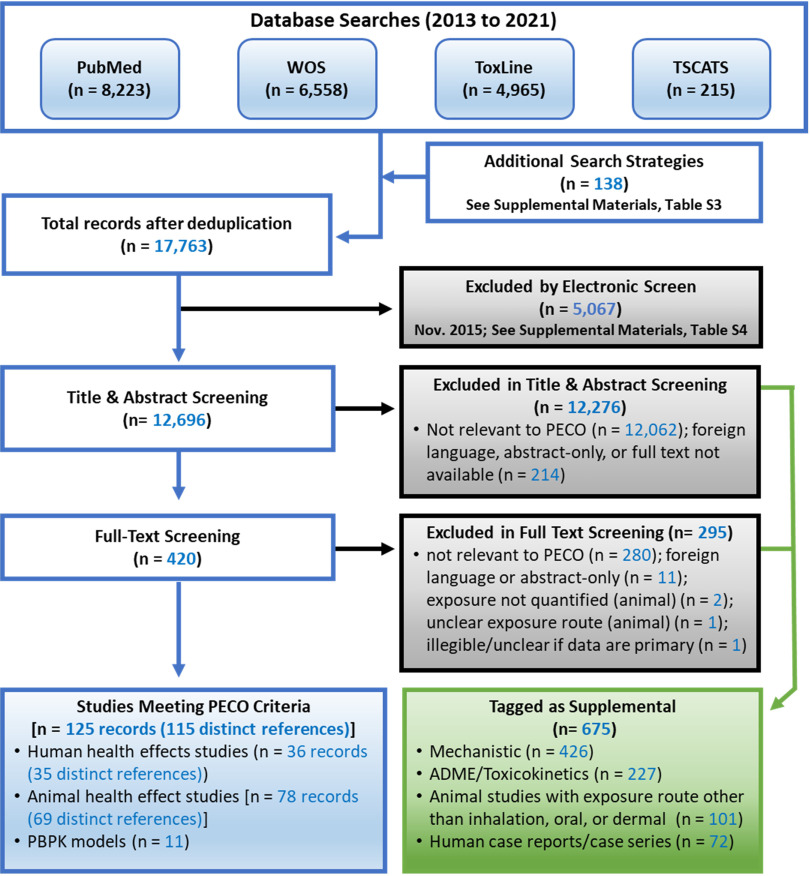
Figure 3.
Literature flow diagram for naphthalene. Multiple records were available for some studies, so the box titled “Studies Meeting PECO Criteria” lists both the total number of records and the number of unique studies. The box titled “Tagged as Supplemental” includes mechanistic studies, ADME/toxicokinetic studies, animal studies with exposure routes other than oral or inhalation (e.g., injection studies), and human case reports. Note: ADME, absorption, distribution, metabolism, and excretion; PBPK models, physiologically based pharmacokinetic models; PECO, populations, exposures, comparators, and outcomes; TSCATS, Toxic Substances Control Act Test Submissions; WOS, Web of Science.