Figure 1. In vitro design and optimization of the lipoMSN for hepatic CRISPR delivery.
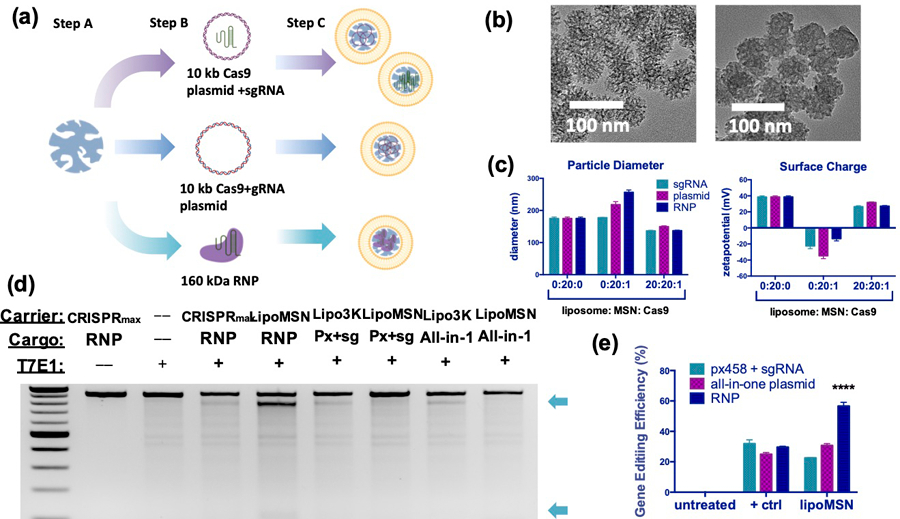
(A) Schematic illustration of lipoMSN loading capabilities spanning small gRNA and larger RNP composed of both Cas9 protein and gRNA. Step A: MSN preparation; step B: loading of CRISPR/Cas9 elements (Cas9 plasmid + gRNA separately, all-in-one Cas9/gRNA plasmid, or Cas9/gRNA RNP); step C: liposome coating to produce a consistent delivery system for various CRIPSR/Cas9 elements. (B) Representative TEM images of MSN (left) and Cas9/gRNA-loaded lipoMSN (right) (C) Size and surface charge characterization of the lipoMSN. (D) Representative gel images obtained from the Surveyor assay for comparison of gene editing efficiency. (E) Semi-quantitative analysis of the gene editing efficiency obtained from the Surveyor assay using Image J software. Results are presented as average ± standard errors of mean (SEM, n = 4). CRISPR/Cas9 elements were delivered in a 1 ug/mL dosage for (D-E). Significance was determined using one-way ANOVA with Tukey’s posthoc test, and represented as **** p < 0.0001.
