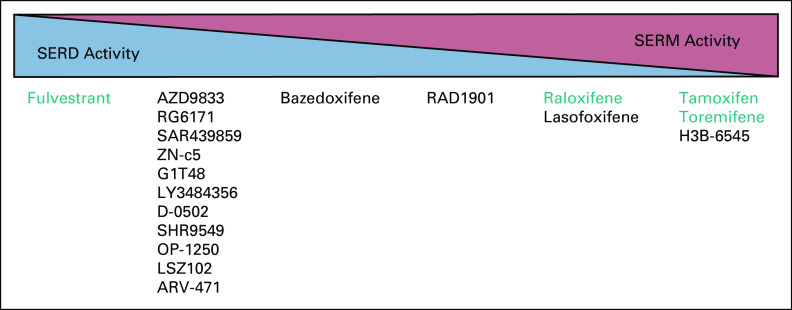
FIG 2.
ER modulators that are currently approved for use in the treatment or prevention of ER-positive breast cancers or that are currently in clinical development. Those drugs (SERDs and SERMs) that are currently approved for clinical use in breast cancer are highlighted in green. Investigational drugs that are currently in development for breast cancer are as follows: AZD9833 (NCT04588298; SERENA-3), RG6171 (giredestrant) (NCT04576455), SAR439859 (amcenestrant) (NCT03284957; AMEERA-1), ZN-c5 (NCT03560531), G1T48 (rintodestrant) (NCT03455270), LY3844356 (NCT04188548; EMBER), D-0502 (NCT03471663), SHR9549 (NCT03596658), OP-1250 (NCT04505826), LSZ102 (NCT02734615), ARV-471 (NCT04072952), bazedoxifene (NCT02448771), RAD1901 (elacestrant) (NCT03778931; EMERALD), lasofoxifene (NCT03781063; ELAINE-1), and H3B-6545 (NCT04288089). The relative SERD or SERM activity reflects the authors' summary of the available literature and may change as more data become available from comparative studies. ER, estrogen receptor; SERD, selective estrogen receptor downregulator or degrader; SERM, selective estrogen receptor modulator.