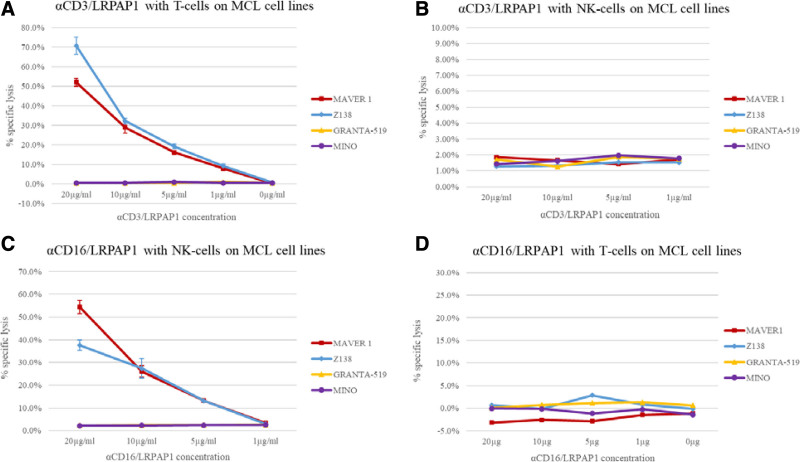
Figure 3.
Bispecific LRPAP1 BAR-bodies—LDH release assays. Isolated T cells and NK cells were used as effector cells. T cells were isolated from PBMCs using the Pan T Cell Isolation Kit, human by Miltenyi Biotech GmbH and cultured for 24 h at 37°C on αCD3/αCD28 coated plates. NK cells were isolated from PBMCs by magnetic depletion of all non-NK cells using the CD56+/CD16+ human NK-Cell Isolation Kit (Miltenyi Biotech GmbH). NK cells were isolated immediately before the ADCC assay without additional activation (eg, by IL-2). Cytotoxic effects of bispecific LRPAP1 BAR-bodies were determined by LDH release. αCD3/LRPAP1 induced specific lysis in LRPAP1-reactive cell lines MAVER1 and Z138 at concentrations of 1.0, 5.0, 10, and 20 µg/mL, respectively. With increasing doses of αCD3/LRPAP1-specific lysis intensified and reached 71% in Z138 and 52% in MAVER1 cells. Granta-519 and Mino cells (not LRPAP1-reactive) were not affected by αCD3/LRPAP1 (A). When the same experiments were repeated using αCD3/LRPAP1 and isolated NK cells, no cytotoxic effects were observed (B). αCD16/LRPAP1 induced cytotoxicity in LRPAP1-reactive MAVER1 and Z138 cells when isolated NK cells were used as effector cells. At 1.0, 5.0, 10, and 20 µg/mL specific lysis reached 3%, 13%, 27%, and 38% in Z138 cells and 3%, 13%, 26%, and 54% in MAVER1 cells. αCD16/LRPAP1 had no effect on Granta-519 and Mino cells (C). When the same experiments were repeated using αCD16/LRPAP1 and isolated T cells, no cytotoxic effects were observed (D). For experiments, 2 × 10E3 lymphoma cells (MAVER1, Z138, Granta-519, Mino) were incubated with αCD3/LRPAP1 and αCD16/LRPAP1 (20/10/5.0/1.0 μg/mL) or PBS and isolated T cells at an E:T ratio of 5:1 (10,000 T cells) or isolated NK cell at an E:T ratio of 2.5:1 (5000 NK cells). Specific lysis was calculated as compared to Triton X-100 controls and is shown in percentage on the y-axis. All experiments were performed in triplicate. BAR=B-cell receptor antigens for reverse targeting; BCR=B-cell receptor.