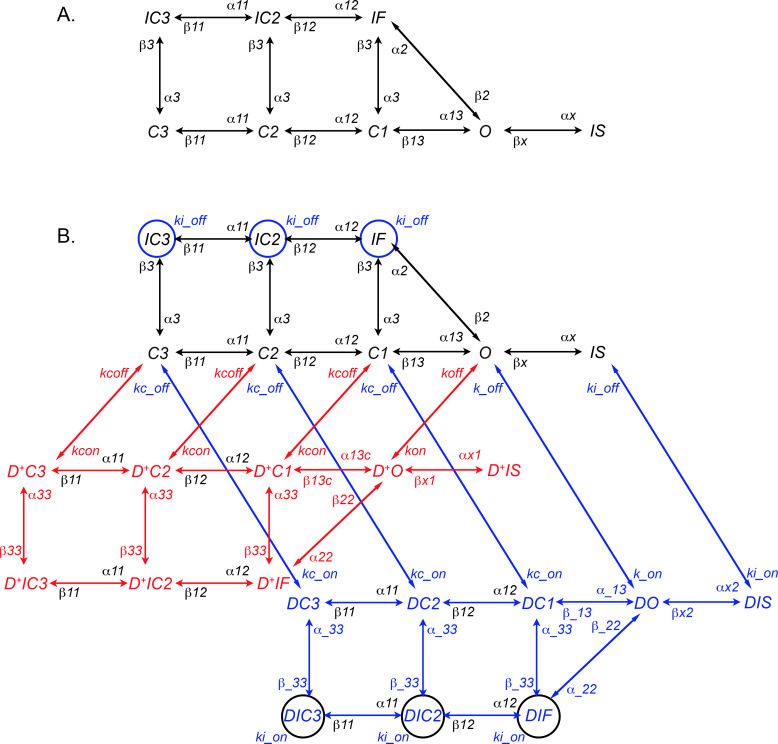
Fig 1. Moreno et al. [4] model of Na+ channel-lidocaine interactions.
(A) The drug-free Na+ channel model. The O state represents the conducting state, while C3, C2, and C1 correspond to 3 closed states. The IC3, IC2, and IF states represent conformational states in which the “fast” inactivation gate is closed, and the IS state represents a state in which a “slow” inactivation gate is closed. Arrows indicate possible conformational state transitions with corresponding voltage-dependent rate constants labeled (e.g., α13 and β13 are the rate constants for transitions from C1 to O and O to C1, respectively). (B) The full lidocaine-Na+ channel interaction model. The drug-free model from (A) is depicted in black. Red (D+ prefix) and blue (D prefix) states represent conformational states where charged and neutral drug is bound, respectively. Charged drug can only bind to non-inactivated states (C3, C2, C1, and O), while neutral drug can bind to any state. Drug binding and unbinding rates are state-dependent, as indicated by the binding and unbinding rates of charged drug to the open state (kon and koff) and closed states (kcon and kcoff), and neutral drug to the open state (k_on and k_off), closed states (kc_on and kc_off), and inactivated states (ki_on and ki_off). For clarity, blue (black) circles as opposed to arrows were used to indicate neutral drug binding (unbinding) to the fast inactivated states with a rate constant ki_on (ki_off).