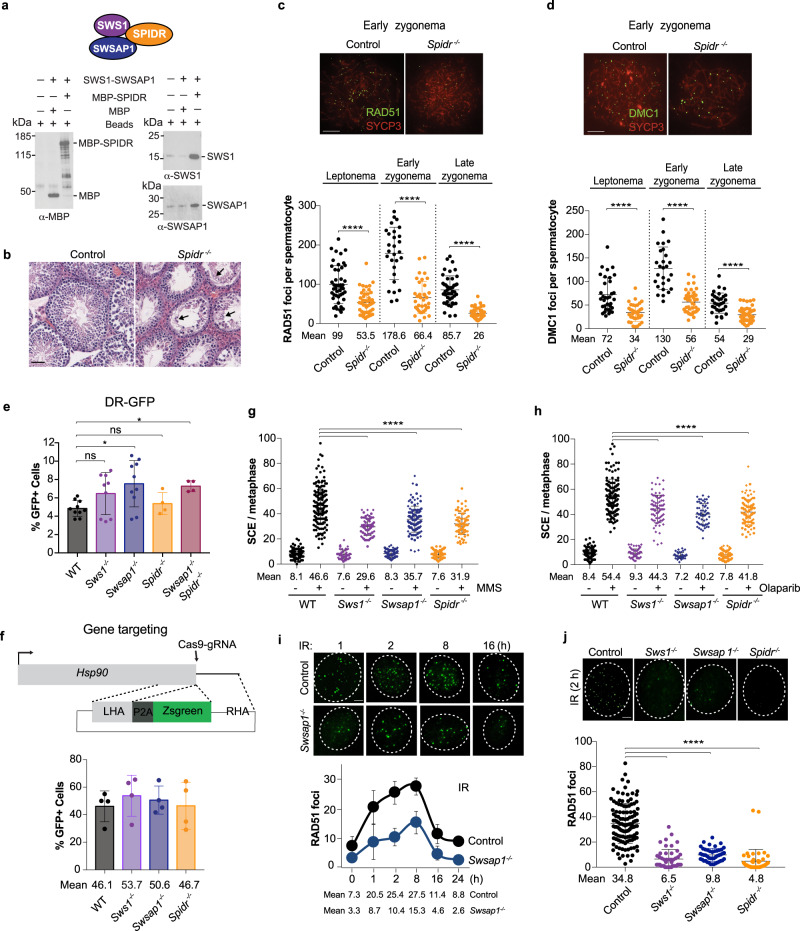
Fig. 1. SWS1–SWSAP1–SPIDR promotes distinct types of HDR.
a SWS1, SWSAP1, and SPIDR form a complex. SPIDR was pulled down with anti-MBP beads and interacting proteins were identified by western blotting with the indicated antibodies. A schematic of the complex is shown on top, the color code for the individual components is maintained in subsequent figures. b Testis section from a Spidr mutant showing seminiferous tubules with substantially reduced post-meiotic germ cells. Arrows indicate infrequent round and elongating spermatids. Scale bar, 50 μm. c, d Representative chromosome spreads from adult mice from early zygonema from control and Spidr mutant spermatocytes to analyze RAD51 and DMC1 focus formation. Foci were counted on the developing chromosome axes that are marked by SYCP3, which at early zygonema form short linear stretches. Scale bar, 10 µm. RAD51 (c) and DMC1 (d) focus formation are substantially reduced in Spidr−/− spermatocytes at early meiotic prophase stages. n = 2. (See also Supplementary Fig. 4e–h). e Sws1−/−, Swsap1−/−, Spidr−/−, and Swsap1−/−Spidr−/− ES cells are proficient in HDR as measured with the DR-GFP reporter. WT n = 14, Sws1−/− n = 9, Swsap1−/− n = 11, Spidr−/− n = 4, and Swsap1−/−Spidr−/− n = 4, where n is the number of independent experiments. (See also Supplementary Fig. 6a). f Sws1−/−, Swsap1−/−, and Spidr−/− ES cells are proficient at DSB-induced gene targeting. Top, schematic of the assay26. A DSB is introduced at the terminus of the Hsp90 gene by Cas9-gRNA. Upon integration of the ZsGreen-coding region through the left and right homology arms (LHA and RHA, respectively) in the circular plasmid, ZsGreen is expressed. Bottom, Gene targeting levels. Values are 4 days after expression of Cas9-gRNA; in the absence of Cas9-gRNA, GFP+ cells are <0.2%. n = 3, where n is the number of independent experiments. g, h SCEs are reduced with Sws1, Swsap1, and Spidr mutation in ES cells after treatment with MMS (0.5 mM for 1 h) (g) and olaparib (20 nM for 17 h) (h). Two independent clones are tested for each mutant, as differentiated by diamonds and circles. g WT-MMS n = 5, WT +MMS n = 6, Sws1−/− -MMS n = 4, Sws1−/− +MMS n = 5, Swsap1−/− -MMS n = 4, Swsap1−/− +MMS n = 5, Spidr−/− -MMS n = 3, Spidr−/− +MMS n = 3. h WT-olaparib n = 6, WT + olaparib n = 7, Sws1−/− -olaparib n = 3, Sws1−/− +olaparib n = 4, Swsap1−/− -olaparib n = 3, Swsap1−/− +olaparib n = 4, Spidr−/− -olaparib n = 2, Spidr−/− +olaparib n = 2, where n is the number of independent experiments. (See also Supplementary Fig. 6c, d). i RAD51 focus formation in Swsap1−/− primary ear fibroblasts is reduced two- to three-fold, but has similar kinetics as control cells when treated with 10 Gy IR. Scale bar, 10 µm. n = 3, where n is the number of independent experiments. j Sws1−/−, Swsap1−/−, and Spidr−/− primary ear fibroblasts have reduced RAD51 focus formation compared with the control cells upon exposure to IR (10 Gy, 2 h). Scale bar, 10 µm. n = 3, where n is the number of independent experiments. Error bars in e–j, mean ± s.d. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; unpaired t test, two-tailed. All source data are provided in the source data file.